Differential behavior of missense mutations in the intersubunit contact domain of the human pyruvate kinase M2 isozyme
- PMID: 19265196
- PMCID: PMC2673266
- DOI: 10.1074/jbc.M808761200
Differential behavior of missense mutations in the intersubunit contact domain of the human pyruvate kinase M2 isozyme
Abstract
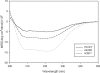
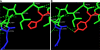
In this study, we attempted to understand the mechanism of regulation of the activity and allosteric behavior of the pyruvate kinase M(2) enzyme and two of its missense mutations, H391Y and K422R, found in cells from Bloom syndrome patients, prone to develop cancer. Results show that despite the presence of mutations in the intersubunit contact domain, the K422R and H391Y mutant proteins maintained their homotetrameric structure, similar to the wild-type protein, but showed a loss of activity of 75 and 20%, respectively. Interestingly, H391Y showed a 6-fold increase in affinity for its substrate phosphoenolpyruvate and behaved like a non-allosteric protein with compromised cooperative binding. However, the affinity for phosphoenolpyruvate was lost significantly in K422R. Unlike K422R, H391Y showed enhanced thermal stability, stability over a range of pH values, a lesser effect of the allosteric inhibitor Phe, and resistance toward structural alteration upon binding of the activator (fructose 1,6-bisphosphate) and inhibitor (Phe). Both mutants showed a slight shift in the pH optimum from 7.4 to 7.0. Although this study signifies the importance of conserved amino acid residues in long-range communications between the subunits of multimeric proteins, the altered behavior of mutants is suggestive of their probable role in tumor-promoting growth and metabolism in Bloom syndrome patients with defective pyruvate kinase M(2).
Figures
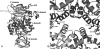





References
-
- Kayne, F. J., and Price, N. C. (1973) Arch. Biochem. Biophys. 159 292–296 - PubMed
-
- Kuo, D. J., O'Connell, E. L., and Rose, I. A. (1979) J. Am. Chem. Soc. 101 5025–5030
-
- Valentini, G., Chiarelli, L., Fortin, R., Speranza, M. L., Galizzi, A., and Mattevi, A. (2000) J. Biol. Chem. 275 18145–18152 - PubMed
-
- Mazurek, S., Boschek, C. B., and Eigenbrodt, E. (1997) J. Bioenerg. Biomembr. 29 315–330 - PubMed
-
- Imamura, K., and Tanaka, T. (1982) Methods Enzymol. 90 150–165 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

