Synthesis of casimiroin and optimization of its quinone reductase 2 and aromatase inhibitory activities
- PMID: 19265439
- PMCID: PMC2673050
- DOI: 10.1021/jm801335z
Synthesis of casimiroin and optimization of its quinone reductase 2 and aromatase inhibitory activities
Abstract
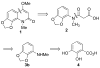
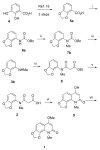
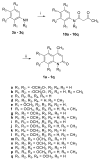
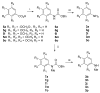
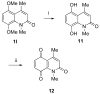
An efficient method has been developed to synthesize casimiroin (1), a component of the edible fruit of Casimiroa edulis, on a multigram scale in good overall yield. The route was versatile enough to provide an array of compound 1 analogues that were evaluated as QR2 and aromatase inhibitors. In addition, X-ray crystallography studies of QR2 in complex with compound 1 and one of its more potent analogues has provided insight into the mechanism of action of this new series of QR2 inhibitors. The initial biological investigations suggest that compound 1 and its analogues merit further investigation as potential chemopreventive or chemotherapeutic agents.
Figures

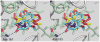
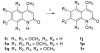
References
-
- Ito A, Shamon LA, Yu B, Mata-Greenwood E, Lee SK, van Breemen RB, Mehta RG, Farnsworth NR, Fong HHS, Pezzuto JM, Kinghorn AD. Antimutagenic Constituents of Casimiroa edulis with Potential Cancer Chemopreventive Activity. J Agric Food Chem. 1998;46:3509–3516.
-
- Jaiswal AK. Human NAD(P)H-Quinone Oxidoreductase(2) - Gene Structure, Activity, and Tissue-Specific Expression. J Biol Chem. 1994;269:14502–14508. - PubMed
-
- Vella F, Gilles FA, Delagrange P, Boutin JA. NRH:Quinone Reductase 2: An Enzyme of Surprises and Mysteries. Biochem Pharmacol. 2005;71:1–12. - PubMed
-
- Harada S, Fujii C, Hayashi A, Ohkoshi N. An Association between Idiopathic Parkinson’s Disease and Polymorphisms of Phase II Detoxification Enzymes: Glutathione S-transferase M1 and Quinone Oxidoreductase 1 and 2. Biochem Biophys Res Commun. 2001;288:887–892. - PubMed
-
- Harada S, Tachikawa H, Kawanishi Y. A Possible Association between an Insertion/Deletion Polymorphism of the NQO2 Gene and Schizophrenia. Psychiatr Genet. 2003;13:205–209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

