Raltegravir, elvitegravir, and metoogravir: the birth of "me-too" HIV-1 integrase inhibitors
- PMID: 19265512
- PMCID: PMC2660292
- DOI: 10.1186/1742-4690-6-25
Raltegravir, elvitegravir, and metoogravir: the birth of "me-too" HIV-1 integrase inhibitors
Erratum in
- Retrovirology. 2009;6:33
Abstract
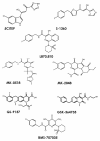
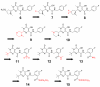
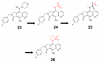
Merck's MK-0518, known as raltegravir, has recently become the first FDA-approved HIV-1 integrase (IN) inhibitor and has since risen to blockbuster drug status. Much research has in turn been conducted over the last few years aimed at recreating but optimizing the compound's interactions with the protein. Resulting me-too drugs have shown favorable pharmacokinetic properties and appear drug-like but, as expected, most have a highly similar interaction with IN to that of raltegravir. We propose that, based upon conclusions drawn from our docking studies illustrated herein, most of these me-too MK-0518 analogues may experience a low success rate against raltegravir-resistant HIV strains. As HIV has a very high mutational competence, the development of drugs with new mechanisms of inhibitory action and/or new active substituents may be a more successful route to take in the development of second- and third-generation IN inhibitors.
Figures

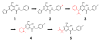



Similar articles
-
Elvitegravir overcomes resistance to raltegravir induced by integrase mutation Y143.AIDS. 2011 Jun 1;25(9):1175-8. doi: 10.1097/QAD.0b013e3283473599. AIDS. 2011. PMID: 21505303 Free PMC article.
-
Design, synthesis and SAR study of bridged tricyclic pyrimidinone carboxamides as HIV-1 integrase inhibitors.Bioorg Med Chem. 2020 Jul 1;28(13):115541. doi: 10.1016/j.bmc.2020.115541. Epub 2020 May 4. Bioorg Med Chem. 2020. PMID: 32389483
-
In vitro resistance selections using elvitegravir, raltegravir, and two metabolites of elvitegravir M1 and M4.Antiviral Res. 2012 Feb;93(2):288-296. doi: 10.1016/j.antiviral.2011.12.008. Epub 2011 Dec 16. Antiviral Res. 2012. PMID: 22197635
-
Diketoacid inhibitors of HIV-1 integrase: from L-708,906 to raltegravir and beyond.Curr Med Chem. 2012;19(8):1177-92. doi: 10.2174/092986712799320565. Curr Med Chem. 2012. PMID: 22214459 Review.
-
Next-generation integrase inhibitors : where to after raltegravir?Drugs. 2013 Mar;73(3):213-28. doi: 10.1007/s40265-013-0015-5. Drugs. 2013. PMID: 23413196 Review.
Cited by
-
Review on fluorinated nucleoside/non-nucleoside FDA-approved antiviral drugs.RSC Adv. 2022 Oct 31;12(48):31032-31045. doi: 10.1039/d2ra05370e. eCollection 2022 Oct 27. RSC Adv. 2022. PMID: 36348998 Free PMC article. Review.
-
Resistance to integrase inhibitors.Viruses. 2010 Jun 25;2(7):1347-66. doi: 10.3390/v2071347. Viruses. 2010. PMID: 20706558 Free PMC article.
-
Evidence for Disruption of Mg2+ Pair as a Resistance Mechanism Against HIV-1 Integrase Strand Transfer Inhibitors.Front Mol Biosci. 2020 Aug 20;7:170. doi: 10.3389/fmolb.2020.00170. eCollection 2020. Front Mol Biosci. 2020. PMID: 32974383 Free PMC article.
-
Design, synthesis and structure-activity studies of rhodanine derivatives as HIV-1 integrase inhibitors.Molecules. 2010 Jun 1;15(6):3958-92. doi: 10.3390/molecules15063958. Molecules. 2010. PMID: 20657419 Free PMC article.
-
Bunyaviridae RNA polymerases (L-protein) have an N-terminal, influenza-like endonuclease domain, essential for viral cap-dependent transcription.PLoS Pathog. 2010 Sep 16;6(9):e1001101. doi: 10.1371/journal.ppat.1001101. PLoS Pathog. 2010. PMID: 20862319 Free PMC article.
References
-
- UNAIDS AIDS epidemic update. 2007. http://data.unaids.org/pub/EPISlides/2007/2007_epiupdate_en.pdf
-
- The Henry J Kaiser Family Foundation The global HIV/AIDS epidemic. HIV/AIDS Policy Fact Sheet. 2007. http://www.kff.org/hivaids/upload/3030-103.pdf
-
- Klimas N, Koneru AO, Fletcher MA. Overview of HIV. Psychosom Med. 1984;70:523–530. - PubMed
-
- Centers for Disease Control and Prevention Using the BED HIV-1 capture EIA assay to estimate incidence using STARHS in the context of surveillance in the United States. 2006. http://data.unaids.org/pub/EPISlides/2006/Statement_BED_Policy_13Dec05_e...
-
- Hammer SM, Eron JJ, Jr, Reiss P, Schooley RT, Thompson MA, Walmsley S, Cahn P, Fischl MA, Gatell JM, Hirsch MS, Jacobsen DM, Montaner JS, Richman DD, Yeni PG, Volberding PA. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society USA panel. JAMA. 2008;300:555–570. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

