In situ real-time chemiluminescence imaging of reactive oxygen species formation from cardiomyocytes
- PMID: 19266051
- PMCID: PMC2650262
- DOI: 10.1155/2008/941729
In situ real-time chemiluminescence imaging of reactive oxygen species formation from cardiomyocytes
Abstract
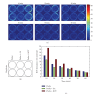
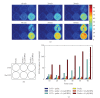
We have applied the highly sensitive chemiluminescence (CL) imaging technique to investigate the in situ ROS formation in cultured monolayers of rat H9c2 cardiomyocytes. Photon emission was detected via an innovative imaging system after incubation of H9c2 cells in culture with luminol and horseradish peroxidase (HRP), suggesting constitutive formation of ROS by the cardiomyocytes. Addition of benzo(a)pyrene-1,6-quinone (BPQ) to cultured H9c2 cells resulted in a 4-5-fold increase in the formation of ROS, as detected by the CL imaging. Both constitutive and BPQ-stimulated CL responses in cultured H9c2 cells were sustained for up to 1 hour. The CL responses were completely abolished in the presence of superoxide dismutase and catalase, suggesting the primary involvement of superoxide and hydrogen peroxide (H2O2). In contrast to BPQ-mediated redox cycling, blockage of mitochondrial electron transport chain by either antimycin A or rotenone exerted marginal effects on the ROS formation by cultured H9c2 cells. Upregulation of cellular antioxidants for detoxifying both superoxide and H2O2 by 3H-1,2-dithiole-3-thione resulted in marked inhibition of both constitutive and BPQ-augmented ROS formation in cultured H9c2 cells. Taken together, we demonstrate the sensitive detection of ROS by CL imaging in cultured cardiomyocytes.
Figures




Similar articles
-
Detection of mitochondria-derived reactive oxygen species production by the chemilumigenic probes lucigenin and luminol.Biochim Biophys Acta. 1999 Jun 28;1428(1):1-12. doi: 10.1016/s0304-4165(99)00040-9. Biochim Biophys Acta. 1999. PMID: 10366754
-
Luminol-amplified chemiluminescence detects mainly superoxide anion produced by human neutrophils.Am J Blood Res. 2017 Jul 25;7(4):41-48. eCollection 2017. Am J Blood Res. 2017. PMID: 28804681 Free PMC article.
-
NADPH Oxidase System Mediates Cholesterol Secoaldehyde-Induced Oxidative Stress and Cytotoxicity in H9c2 Cardiomyocytes.2022 Sep 21. In: Parinandi NL, Hund TJ, editors. Cardiovascular Signaling in Health and Disease [Internet]. Cham (CH): Springer; 2022. 2022 Sep 21. In: Parinandi NL, Hund TJ, editors. Cardiovascular Signaling in Health and Disease [Internet]. Cham (CH): Springer; 2022. PMID: 37988553 Free Books & Documents. Review.
-
Induction of cellular glutathione-linked enzymes and catalase by the unique chemoprotective agent, 3H-1,2-dithiole-3-thione in rat cardiomyocytes affords protection against oxidative cell injury.Pharmacol Res. 2002 Jun;45(6):491-7. doi: 10.1006/phrs.2002.0991. Pharmacol Res. 2002. PMID: 12162951
-
Peroxidase-catalyzed chemiluminescence system and its application in immunoassay.Talanta. 2018 Apr 1;180:260-270. doi: 10.1016/j.talanta.2017.12.024. Epub 2017 Dec 14. Talanta. 2018. PMID: 29332809 Review.
Cited by
-
A Highly Sensitive Chemiluminometric Assay for Real-Time Detection of Biological Hydrogen Peroxide Formation.React Oxyg Species (Apex). 2016 May;1(3):216-227. doi: 10.20455/ros.2016.841. React Oxyg Species (Apex). 2016. PMID: 29780884 Free PMC article.
References
-
- Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury—part I: basic mechanisms and in vivo monitoring of ROS. Circulation. 2003;108(16):1912–1916. - PubMed
-
- Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury—part II: animal and human studies. Circulation. 2003;108(17):2034–2040. - PubMed
-
- Kehrer JP. Free radicals as mediators of tissue injury and disease. Critical Review in Toxicology. 1993;23(1):21–48. - PubMed
-
- Li Y, Zhu H, Stansbury KH, Trush MA. Role of reactive oxygen species in multistage carcinogenesis. In: Thomas CE, Kalyanaraman B, editors. Oxygen Radicals and the Disease Process. Amsterdam, The Netherlands: Harwood Academic; 1997. pp. 237–277.
-
- Jia Z, Zhu H, Misra BR, Mahaney JE, Li Y, Misra HP. EPR studies on the superoxide-scavenging capacity of the nutraceutical resveratrol. Molecular and Cellular Biochemistry. 2008;313(1-2):187–194. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

