Oral bisphosphonates and risk of atrial fibrillation and flutter in women: a self-controlled case-series safety analysis
- PMID: 19266096
- PMCID: PMC2648983
- DOI: 10.1371/journal.pone.0004720
Oral bisphosphonates and risk of atrial fibrillation and flutter in women: a self-controlled case-series safety analysis
Abstract
Background: A recent trial unexpectedly reported that atrial fibrillation, when defined as serious, occurred more often in participants randomized to an annual infusion of the relatively new parenteral bisphosphonate, zoledronic acid, than among those given placebo, but had limited power. Two subsequent population-based case-control studies of patients receiving a more established oral bisphosphonate, alendronic acid, reported conflicting results, possibly due to uncontrolled confounding factors.
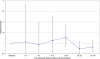
Methodology/principal findings: We used the United Kingdom General Practice Research Database to assess the risk of atrial fibrillation and flutter in women exposed to the oral bisphosphonates, alendronic acid and risedronate sodium. The self-controlled case-series method was used to minimise the potential for confounding. The age-adjusted incidence rate ratio for atrial fibrillation or flutter in individuals during their exposure to these oral bisphosphonates (n = 2195) was 1.07 (95% CI 0.94-1.21). The age-adjusted incidence rate ratio for alendronic acid (n = 1489) and risedronate sodium (n = 649) exposed individuals were 1.09 (95% CI 0.93-1.26) and 0.99 (95% CI 0.78-1.26) respectively. In post-hoc analyses, an increased risk of incident atrial fibrillation or flutter was detected for patients during their first few months of alendronic acid therapy.
Conclusions/significance: We found no robust evidence of an overall long-term increased risk of atrial fibrillation or flutter associated with continued exposure to the oral bisphosphonates, alendronic acid and risedronate sodium. A possible signal for an increase in risk during the first few months of therapy with alendronic acid needs to be re-assessed in additional studies.
Conflict of interest statement
Figures




References
-
- Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007 May 3;356(18):1809–22. - PubMed
-
- Cummings SR, Schwartz AV, Black DM. Alendronate and atrial fibrillation. N Engl J Med. 2007 May 3;356(18):1895–6. - PubMed
-
- Heckbert SR, Li G, Cummings SR, Smith NL, Psaty BM. Use of alendronate and risk of incident atrial fibrillation in women. Arch Intern Med. 2008 Apr 28;168(8):826–31. - PubMed
-
- Karam R, Camm J, McClung M. Yearly zoledronic acid in postmenopausal osteoporosis. N Engl J Med. 2007 Aug 16;357(7):712–3. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

