Multipotent stromal cells are activated to reduce apoptosis in part by upregulation and secretion of stanniocalcin-1
- PMID: 19267325
- PMCID: PMC4742302
- DOI: 10.1002/stem.20080742
Multipotent stromal cells are activated to reduce apoptosis in part by upregulation and secretion of stanniocalcin-1
Abstract
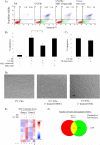
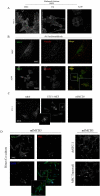
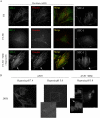
Multipotent stromal cells (MSCs) have been shown to reduce apoptosis in injured cells by secretion of paracrine factors, but these factors were not fully defined. We observed that coculture of MSCs with previously UV-irradiated fibroblasts reduced apoptosis of the irradiated cells, but fresh MSC conditioned medium was unable reproduce the effect. Comparative microarray analysis of MSCs grown in the presence or absence of UV-irradiated fibroblasts demonstrated that the MSCs were activated by the apoptotic cells to increase synthesis and secretion of stanniocalcin-1 (STC-1), a peptide hormone that modulates mineral metabolism and has pleiotrophic effects that have not been fully characterized. We showed that STC-1 was required but not sufficient for reduction of apoptosis of UV-irradiated fibroblasts. In contrast, we demonstrated that MSC-derived STC-1 was both required and sufficient for reduction of apoptosis of lung cancer epithelial cells made apoptotic by incubation at low pH in hypoxia. Our data demonstrate that STC-1 mediates the antiapoptotic effects of MSCs in two distinct models of apoptosis in vitro.
Figures



References
-
- Owen M, Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Foundation symposium. 1988;136:42–60. - PubMed
-
- Owen M. Marrow stromal stem cells. Journal of cell science. 1988;10:63–76. - PubMed
-
- Chopp M, Zhang XH, Li Y, et al. Spinal cord injury in rat: treatment with bone marrow stromal cell transplantation. Neuroreport. 2000;11:3001–3005. - PubMed
-
- Horwitz EM, Gordon PL, Koo WK, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:8932–8937. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

