Synthesis of 1,3-diamines through rhodium-catalyzed C-H insertion
- PMID: 19267373
- PMCID: PMC2822642
- DOI: 10.1002/anie.200806192
Synthesis of 1,3-diamines through rhodium-catalyzed C-H insertion
Abstract
A grand opening: N-Boc-N-alkylsulfamides are effective substrates for the title transformation. Oxidative cyclization is highly chemoselective as well as being both stereospecific and diastereoselective. With the advent of new protocols that facilitate ring opening of the six-membered-ring heterocyclic products, access to differentially protected 1,3-diamines has been made possible (see scheme).
Figures
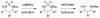
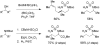
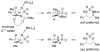
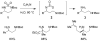

References
-
- Espino CG, Du Bois J. In: Modern Rhodium-Catalyzed Organic Reactions. Evans PA, editor. Wiley-VCH; Weinheim: 2005. pp. 379–416.
- Dáaz-Requejo MM, Pérez PJ. Chem. Rev. 2008;108:3379–3394. - PubMed
- Davies HML, Manning JR. Nature. 2008;451:417–424. - PMC - PubMed
- Lebel H, Leogane O, Huard K, Lectard S. Pure Appl. Chem. 2006;78:363–375.
- Halfen JA. Curr. Org. Chem. 2005;9:657–669.
- Dauban P, Dodd RH. Synlett. 2003:1571–1586.
-
- Kim M, Mulcahy JV, Espino CG, Du Bois J. Org. Lett. 2006;8:1073–1076. - PubMed
- Du Bois J. Chemtracts: Org. Chem. 2005;18:1–13. and references therein.
-
-
For some leading references of amination reactions with dinuclear rhodium catalysts, see: Huard K, Lebel H. Chem. Eur. J. 2008;14:6222–6230. Shen M, Leslie BE, Driver TG. Angew. Chem. Angew. Chem. Int. Ed. 2008;2008;12047:5134–5137. 5056–5059. Morin MST, Toumieux S, Compain P, Peyrat S, Kalinowska-Tluscik J. Tetrahedron Lett. 2007;48:8531–8535. Reddy RP, Davies HML. Org. Lett. 2006;8:5013–5016. Padwa A, Flick AC, Leverett CA, Stengel T. J. Org. Chem. 2004;69:6377–6386. Fruit C, Müller P. Helv. Chim. Acta. 2004;87:1607–1615. Liang J-L, Yuan S-X, Chan PWH, Che C-M. Tetrahedron Lett. 2003;44:5917–5920.
-
-
-
One example of an intramolecular alkylsulfamide C—H insertion reaction was shown in a prior report, see: Espino CG, Fiori KW, Kim M, Du Bois J. J. Am. Chem. Soc. 2004;126:15378–15379.
-
-
-
For metal-catalyzed diamination of alkenes using sulfamides, see: Muñiz K, Streuff J, Hövelmann CH, Núñez A. Angew. Chem. 2007;119:7255–7258. Angew. Chem. Int. Ed. 2007, 46, 7125–7127. Zabawa TP, Kasi D, Chemler SR. J. Am. Chem. Soc. 2005;127:11250–11251.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

