Climbing nitrogenase: toward a mechanism of enzymatic nitrogen fixation
- PMID: 19267458
- PMCID: PMC2684573
- DOI: 10.1021/ar8002128
Climbing nitrogenase: toward a mechanism of enzymatic nitrogen fixation
Abstract
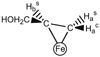
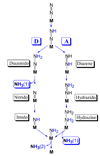
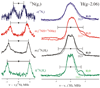
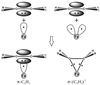
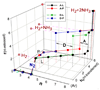
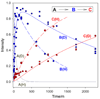
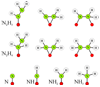
"Nitrogen fixation", the reduction of dinitrogen (N2) to two ammonia (NH3) molecules, by the Mo-dependent nitrogenase is essential for all life. Despite four decades of research, a daunting number of unanswered questions about the mechanism of nitrogenase activity make it the "Everest of enzymes". This Account describes our efforts to climb one "face" of this mountain by meeting two interdependent challenges central to determining the mechanism of biological N2 reduction. The first challenge is to determine the reaction pathway: the composition and structure of each of the substrate-derived moieties bound to the catalytic FeMo cofactor (FeMo-co) of the molybdenum-iron (MoFe) protein of nitrogenase. To overcome this challenge, it is necessary to discriminate between the two classes of potential reaction pathways: (1) a "distal" (D) pathway, in which H atoms add sequentially at a single N or (2) an "alternating" (A) pathway, in which H atoms add alternately to the two N atoms of N2. Second, it is necessary to characterize the dynamics of conversion among intermediates within the accepted Lowe-Thorneley kinetic scheme for N2 reduction. That goal requires an experimental determination of the number of electrons and protons delivered to the MoFe protein as well as their "inventory", a partition into those residing on each of the reaction components and released as H2 or NH3. The principal obstacle to this "climb" has been the inability to generate N2 reduction intermediates for characterization. A combination of genetic, biochemical, and spectroscopic approaches recently overcame this obstacle. These experiments identified one of the four-iron Fe-S faces of the active-site FeMo-co as the specific site of reactivity, indicated that the side chain of residue alpha70V controls access to this face, and supported the involvement of the side chain of residue alpha195H in proton delivery. We can now freeze-quench trap N2 reduction pathway intermediates and use electron-nuclear double resonance (ENDOR) and electron spin-echo envelope modulation (ESEEM) spectroscopies to characterize them. However, even successful trapping of a N2 reduction intermediate occurs without synchronous electron delivery to the MoFe protein. As a result, the number of electrons and protons, n, delivered to MoFe during its formation is unknown. To determine n and the electron inventory, we initially employed ENDOR spectroscopy to analyze the substrate moiety bound to the FeMo-co and 57Fe within the cofactor. Difficulties in using that approach led us to devise a robust kinetic protocol for determining n of a trapped intermediate. This Account describes strategies that we have formulated to bring this "face" of the nitrogenase mechanism into view and afford approaches to its climb. Although the summit remains distant, we look forward to continued progress in the ascent.
Figures


References
-
- Zhao Y, Bian S-M, Zhou H-N, Huang J-F. Diversity of nitrogenase systems in diazotrophs. Journal of Integrative Plant Biology. 2006;48:745–755.
-
- Burgess BK, Lowe DL. Mechanism of Molybdenum Nitrogenase. Chem. Rev. 1996;96:2983–3011. - PubMed
-
- Thorneley RNF, Lowe DJ. In: Molybdenum Enzymes. Spiro TG, editor. Vol. 7. New York: Wiley-Interscience; 1985. pp. 89–116.
-
- Wilson PE, Nyborg AC, Watt GD. Duplication and extension of the Thorneley and Lowe kinetic model for Klebsiella pneumoniae nitrogenase catalysis using a MATHEMATICA software platform. Biophys. Chem. 2001;91:281–304. - PubMed
-
- Rees DC, Tezcan FA, Haynes CA, Walton MY, Andrade S, Einsle O, Howard JB. Structural basis of biological nitrogen fixation. Phil. Trans. R. Soc. A. 2005;363:971–984. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

