The high affinity selectin glycan ligand C2-O-sLex and mRNA transcripts of the core 2 beta-1,6-N-acetylglucosaminyltransferase (C2GnT1) gene are highly expressed in human colorectal adenocarcinomas
- PMID: 19267921
- PMCID: PMC2662873
- DOI: 10.1186/1471-2407-9-79
The high affinity selectin glycan ligand C2-O-sLex and mRNA transcripts of the core 2 beta-1,6-N-acetylglucosaminyltransferase (C2GnT1) gene are highly expressed in human colorectal adenocarcinomas
Abstract
Background: The metastasis of cancer cells and leukocyte extravasation into inflamed tissues share common features. Specialized carbohydrates modified with sialyl Lewis x (sLex) antigens on leukocyte membranes are ligands for selectin adhesion molecules on activated vascular endothelial cells at inflammatory sites. The activity of the enzyme core 2 beta1,6 N-acetylglucosaminyltransferase (C2GnT1) in leukocytes greatly increases their ability to bind to endothelial selectins. C2GnT1 is essential for the synthesis of core 2-branched O-linked carbohydrates terminated with sLex (C2-O-sLex). Our goal was to determine the expression profiles of C2-O-sLex in the malignant progression and metastasis of colorectal adenocarcinomas. The well characterized CHO-131 monoclonal antibody (mAb) specifically recognizes C2-O-sLex present in human leukocytes and carcinoma cells. Using CHO-131 mAb, we investigated whether C2-O-sLex was present in 113 human primary colorectal adenocarcinomas, 10 colorectal adenomas, 46 metastatic liver tumors, 28 normal colorectal tissues, and 5 normal liver tissues by immunohistochemistry. We also examined mRNA levels of the enzyme core 2 beta1,6-N-acetylglucosaminyltransferase (C2GnT1) in 20 well, 15 moderately, and 2 poorly differentiated colorectal adenocarcinomas, and in 5 normal colorectal tissues by using quantitative real-time polymerase chain reactions (RT-PCR).
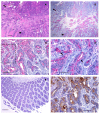
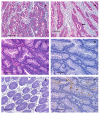
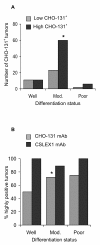
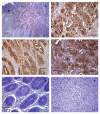
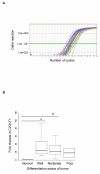
Results: We observed high reactivity with CHO-131 mAb in approximately 70% of colorectal carcinomas and 87% of metastatic liver tumors but a lack of reactivity in colorectal adenomas and normal colonic and liver tissues. Positive reactivity with CHO-131 mAb was very prominent in neoplastic colorectal glands of well to moderately differentiated adenocarcinomas. The most intense staining with CHO-131 mAb was observed at the advancing edge of tumors with the deepest invasive components.Finally, we analyzed C2GnT1 mRNA levels in 37 colorectal adenocarcinomas and 5 normal colorectal tissues by RT-PCR. Significantly, we observed a greater than 15-fold increase in C2GnT1 mRNA levels in colorectal adenocarcinomas compared to normal colorectal tissues.
Conclusion: C2-O-sLex, detected by the CHO-131 mAb, is a tumor associated antigen whose expression is highly upregulated in colorectal adenocarcinomas and metastatic liver tumors compared to normal tissues. C2-O-sLex is a potentially useful early predictor of metastasis.
Figures

Similar articles
-
C2-O-sLeX glycoproteins are E-selectin ligands that regulate invasion of human colon and hepatic carcinoma cells.PLoS One. 2011 Jan 19;6(1):e16281. doi: 10.1371/journal.pone.0016281. PLoS One. 2011. PMID: 21283832 Free PMC article.
-
Expression of the high-affinity selectin glycan ligand C2-O-sLeX by colon carcinoma cells.Cancer Lett. 2005 Jan 10;217(1):105-13. doi: 10.1016/j.canlet.2004.06.038. Cancer Lett. 2005. PMID: 15596301
-
A potential role for 6-sulfo sialyl Lewis X in metastasis of bladder urothelial carcinoma.Urol Oncol. 2015 Nov;33(11):496.e1-9. doi: 10.1016/j.urolonc.2015.05.026. Epub 2015 Jun 29. Urol Oncol. 2015. PMID: 26137907
-
The biology of E-selectin ligands in leukemogenesis.Adv Cancer Res. 2023;157:229-250. doi: 10.1016/bs.acr.2022.07.001. Epub 2022 Sep 29. Adv Cancer Res. 2023. PMID: 36725110 Review.
-
Prognostic value of carcinoembryonic antigen in colorectal adenocarcinoma: expanding hypotheses into clinical practice.Clin Exp Med. 2025 Jan 3;25(1):30. doi: 10.1007/s10238-024-01547-1. Clin Exp Med. 2025. PMID: 39753986 Free PMC article.
Cited by
-
Glycosylation potential of human prostate cancer cell lines.Glycoconj J. 2012 Oct;29(7):525-37. doi: 10.1007/s10719-012-9428-8. Epub 2012 Jul 28. Glycoconj J. 2012. PMID: 22843320 Free PMC article.
-
A Systematic Review on the Implications of O-linked Glycan Branching and Truncating Enzymes on Cancer Progression and Metastasis.Cells. 2020 Feb 14;9(2):446. doi: 10.3390/cells9020446. Cells. 2020. PMID: 32075174 Free PMC article.
-
Specific (sialyl-)Lewis core 2 O-glycans differentiate colorectal cancer from healthy colon epithelium.Theranostics. 2022 May 26;12(10):4498-4512. doi: 10.7150/thno.72818. eCollection 2022. Theranostics. 2022. PMID: 35832079 Free PMC article.
-
Leveraging fluorinated glucosamine action to boost antitumor immunity.Curr Opin Immunol. 2013 Apr;25(2):206-13. doi: 10.1016/j.coi.2012.11.003. Epub 2012 Dec 6. Curr Opin Immunol. 2013. PMID: 23219268 Free PMC article. Review.
-
Bisimidazolium Salt Glycosyltransferase Inhibitors Suppress Hepatocellular Carcinoma Progression In Vitro and In Vivo.Pharmaceuticals (Basel). 2022 Jun 5;15(6):716. doi: 10.3390/ph15060716. Pharmaceuticals (Basel). 2022. PMID: 35745636 Free PMC article.
References
-
- Hakomori S. Tumor malignancy defined by aberrant glycosylation and sphingo(glyco)lipid metabolism. Cancer Res. 1996;56(23):5309–5318. - PubMed
-
- Irimura T, Nakamori S, Matsushita Y, Taniuchi Y, Todoroki N, Tsuji T, Izumi Y, Kawamura Y, Hoff SD, Cleary KR, Ota DM. Colorectal cancer metastasis determined by carbohydrate-mediated cell adhesion: role of sialyl-Lex antigens. Semin Cancer Biol. 1993;4(5):319–324. - PubMed
-
- Nakamori S, Kameyama M, Imaoka S, Furukawa H, Ishikawa O, Sasaki Y, Kabuto T, Iwanaga T, Matsushita Y, Irimura T. Increased expression of sialyl Lewisx antigen correlates with poor survival in patients with colorectal carcinoma: clinicopathological and immunohistochemical study. Cancer Res. 1993;53(15):3632–3637. - PubMed
-
- Kishimoto T, Ishikura H, Kimura C, Takahashi T, Kato H, Yoshiki T. Phenotypes correlating to metastatic properties of pancreas adenocarcinoma in vivo: the importance of surface sialyl Lewis(a) antigen. Int J Cancer. 1996;69(4):290–294. doi: 10.1002/(SICI)1097-0215(19960822)69:4<290::AID-IJC9>3.0.CO;2-S. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

