Cloning-free regulated monitoring of reporter and gene expression
- PMID: 19267938
- PMCID: PMC2662838
- DOI: 10.1186/1471-2199-10-20
Cloning-free regulated monitoring of reporter and gene expression
Abstract
Background: The majority of the promoters, their regulatory elements, and their variations in the human genome remain unknown. Reporter gene technology for transcriptional activity is a widely used tool for the study of promoter structure, gene regulation, and signaling pathways. Construction of transcriptional reporter vectors, including use of cis-acting sequences, requires cloning and time-demanding manipulations, particularly with introduced mutations.
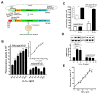
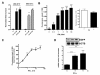
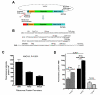
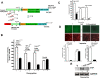
Results: In this report, we describe a cloning-free strategy to generate transcriptionally-controllable linear reporter constructs. This approach was applied in common transcriptional models of inflammatory response and the interferon system. In addition, it was used to delineate minimal transcriptional activity of selected ribosomal protein promoters. The approach was tested for conversion of genes into TetO-inducible/repressible expression cassettes.
Conclusion: The simple introduction and tuning of any transcriptional control in the linear DNA product renders promoter activation and regulated gene studies simple and versatile.
Figures

Similar articles
-
Promoter Boundaries for the luxCDABE and betIBA-proXWV Operons in Vibrio harveyi Defined by the Method Rapid Arbitrary PCR Insertion Libraries (RAIL).J Bacteriol. 2018 May 9;200(11):e00724-17. doi: 10.1128/JB.00724-17. Print 2018 Jun 1. J Bacteriol. 2018. PMID: 29531178 Free PMC article.
-
Making reporter gene constructs to analyze cis-regulatory elements.Methods Mol Biol. 2011;772:397-408. doi: 10.1007/978-1-61779-228-1_23. Methods Mol Biol. 2011. PMID: 22065451
-
Identification of a novel transferable cis element in the promoter of an estrogen-responsive gene that modulates sensitivity to hormone and antihormone.Mol Endocrinol. 1997 Mar;11(3):330-41. doi: 10.1210/mend.11.3.9899. Mol Endocrinol. 1997. PMID: 9058379
-
In vivo molecular-genetic imaging.J Cell Biochem Suppl. 2002;39:172-83. doi: 10.1002/jcb.10433. J Cell Biochem Suppl. 2002. PMID: 12552617 Review.
-
Transcriptional target-based expression cloning of immunoregulatory molecules.Immunol Res. 2010 Jul;47(1-3):172-8. doi: 10.1007/s12026-009-8148-z. Immunol Res. 2010. PMID: 20069388 Free PMC article. Review.
Cited by
-
UU/UA dinucleotide frequency reduction in coding regions results in increased mRNA stability and protein expression.Mol Ther. 2012 May;20(5):954-9. doi: 10.1038/mt.2012.29. Epub 2012 Mar 20. Mol Ther. 2012. PMID: 22434136 Free PMC article.
-
Identification of a novel genetic locus underlying tremor and dystonia.Hum Genomics. 2017 Nov 6;11(1):25. doi: 10.1186/s40246-017-0123-5. Hum Genomics. 2017. PMID: 29110692 Free PMC article.
-
Green fluorescent protein reporter system with transcriptional sequence heterogeneity for monitoring the interferon response.J Virol. 2011 Sep;85(18):9268-75. doi: 10.1128/JVI.00772-11. Epub 2011 Jul 13. J Virol. 2011. PMID: 21752918 Free PMC article.
-
A novel mechanism for variable phenotypic expressivity in Mendelian diseases uncovered by an AU-rich element (ARE)-creating mutation.Genome Biol. 2017 Jul 28;18(1):144. doi: 10.1186/s13059-017-1274-3. Genome Biol. 2017. PMID: 28754144 Free PMC article.
-
Beyond the exome: utility of long-read whole genome sequencing in exome-negative autosomal recessive diseases.Genome Med. 2023 Dec 14;15(1):114. doi: 10.1186/s13073-023-01270-8. Genome Med. 2023. PMID: 38098057 Free PMC article.
References
-
- Blanchette M, Bataille AR, Chen X, Poitras C, Laganiere J, Lefebvre C, Deblois G, Giguere V, Ferretti V, Bergeron D, et al. Genome-wide computational prediction of transcriptional regulatory modules reveals new insights into human gene expression. Genome Res. 2006;16:656–668. doi: 10.1101/gr.4866006. - DOI - PMC - PubMed
-
- Jegga AG, Gupta A, Gowrisankar S, Deshmukh MA, Connolly S, Finley K, Aronow BJ. CisMols Analyzer: identification of compositionally similar cis-element clusters in ortholog conserved regions of coordinately expressed genes. Nucleic Acids Res. 2005:W408–411. doi: 10.1093/nar/gki486. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

