PALB2 links BRCA1 and BRCA2 in the DNA-damage response
- PMID: 19268590
- PMCID: PMC2750839
- DOI: 10.1016/j.cub.2009.02.018
PALB2 links BRCA1 and BRCA2 in the DNA-damage response
Abstract
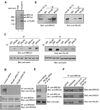
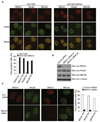
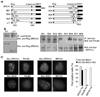
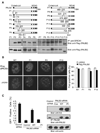
BRCA1 and BRCA2 are often mutated in familial breast and ovarian cancer. Both tumor suppressors play key roles in the DNA-damage response. However, it remains unclear whether these two tumor suppressor function together in the same DNA-damage response pathway. Here, we show that BRCA1 associates with BRCA2 through PALB2/FANCN, a major binding partner of BRCA2. The interaction between BRCA1 and BRCA2 is abrogated in PALB2-deficient Fanconi anemia cells and in the cells depleted of PALB2 by small interfering RNA. Moreover, we show that BRCA1 promotes the concentration of PALB2 and BRCA2 at DNA-damage sites and the interaction between BRCA1 and PALB2 is important for the homologous recombination repair. Taken together, our results indicate that BRCA1 is an upstream regulator of BRCA2 in the DNA-damage response, and PALB2 is the linker between BRCA1 and BRCA2.
Figures
References
-
- Venkitaraman AR. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell. 2002;108:171–182. - PubMed
-
- Xia B, Sheng Q, Nakanishi K, Ohashi A, Wu J, Christ N, Liu X, Jasin M, Couch FJ, Livingston DM. Control of BRCA2 cellular and clinical functions by a nuclear partner, PALB2. Mol. Cell. 2006;22:719–729. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

