Sex differences in the response to activation of the poly (ADP-ribose) polymerase pathway after experimental stroke
- PMID: 19268668
- PMCID: PMC2672307
- DOI: 10.1016/j.expneurol.2009.02.012
Sex differences in the response to activation of the poly (ADP-ribose) polymerase pathway after experimental stroke
Abstract
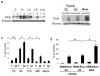
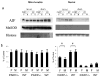
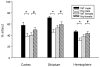
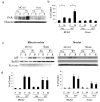
It is increasingly recognized that histological and functional outcomes after stroke are shaped by biologic sex. Emerging data suggests that ischemic cell death pathways are sexually dimorphic (Hurn, P., Vannucci, S., Hagberg, H. (2005) Adult or perinatal brain injury: does sex matter?. Stroke 36, 193-195 ; Lang, J.T., McCullough, L.D. (2008) Pathways to ischemic neuronal cell death: are sex differences relevant?. J. Transl. Med. 6). Reducing neuronal nitric oxide (NO) or poly-ADP-ribose polymerase (PARP1) activation protects only the male brain (Hagberg, H., et al. PARP-1 gene disruption in mice preferentially protects males from perinatal brain injury. J. Neurochem. 90, 1068-1075 (2004)), and paradoxically enhances ischemic injury in females (McCullough, L.D., et al. Ischemic nitric oxide and poly (ADP-ribose) polymerase-1 in cerebral ischemia: male toxicity, female protection. J. Cereb. Blood Flow Metab. 25, 502-512 (2005)). In this study, we examined downstream mediators of NO/PARP activation to investigate possible mediators of ischemic sexual dimorphism. Nuclear translocation of Apoptosis Inducing Factor (AIF) was equivalent in wild type males and females after stroke and was unaffected by estrogen exposure. Deletion of PARP1 led to a dramatic reduction in stroke-induced poly (ADP-ribose) polymerase (PAR) formation and AIF translocation in both sexes, yet ischemic damage was reduced only in males. Subsequent examination of AIF-deficient Harlequin mice demonstrated that male Harlequin mice had less PAR formation, reduced AIF translocation and less ischemic damage than male wild type mice. In contrast, female Harlequin mice had no neuroprotective effect of gene deletion despite robust reductions in PAR formation and AIF translocation. Although equivalent activation of this cell death pathway occurs in both sexes after ischemia, detrimental effects are only present in males. AIF translocation and PAR formation do not mediate ischemic injury in the female brain, therefore agents designed to reduce PARP1 activation are unlikely to benefit females.
Figures



References
-
- Alkayed N, et al. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159–165. - PubMed
-
- Bredt DS, et al. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990;347:768–70. - PubMed
-
- Cao G, et al. Translocation of apoptosis-inducing factor in vulnerable neurons after transient cerebral ischemia and in neuronal cultures after oxygen-glucose deprivation. J Cereb Blood Flow Metab. 2003;23:1137–1150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

