Quantum dots thermal stability improves simultaneous phenotype-specific telomere length measurement by FISH-flow cytometry
- PMID: 19268672
- PMCID: PMC2752384
- DOI: 10.1016/j.jim.2009.02.004
Quantum dots thermal stability improves simultaneous phenotype-specific telomere length measurement by FISH-flow cytometry
Abstract
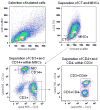
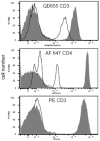
Telomere length analysis has been greatly simplified by the quantitative flow cytometry technique FISH-flow. In this method, a fluorescein-labeled synthetic oligonucleotide complementary to the telomere terminal repeat sequence is hybridized to the telomere sequence and the resulting fluorescence measured by flow cytometry. This technique has supplanted the traditional laborious Southern blot telomere length measurement techniques in many laboratories, and allows single cell analysis of telomere length in high-throughput sample formats. Nevertheless, the harsh conditions required for telomere probe annealing (82 degrees C) has made it difficult to successfully combine this technique with simultaneous immunolabeling. Most traditional organic fluorescent probes (i.e. fluorescein, phycoerythrin, etc.) have limited thermal stability and do not survive the high temperature annealing process, despite efforts to covalently crosslink the antigen-antibody-fluorophore complex. This loss of probe fluorescence has made it difficult to measure FISH-flow in complex lymphocyte populations, and has generally forced investigators to use fluorescent-activated cell sorting to pre-separate their populations, a laborious technique that requires prohibitively large numbers of cells. In this study, we have substituted quantum dots (nanoparticles) for traditional fluorophores in FISH-flow. Quantum dots were demonstrated to possess much greater thermal stability than traditional low molecular weight and phycobiliprotein fluorophores. Quantum dot antibody conjugates directed against monocyte and T cell antigens were found to retain most of their fluorescence following the high temperature annealing step, allowing simultaneous fluorescent immunophenotyping and telomere length measurement. Since quantum dots have very narrow emission bandwidths, we were able to analyze multiple quantum dot antibody conjugates (Qdot 605, 655 and 705) simultaneously with FISH-flow measurement to assess the age-associated decline in telomere length in both human monocytes and T cell subsets. With quantum dot immunolabeling, the mean decrease rate in telomere length for CD4+ cells was calculated at 41.8 bp/year, very close to previously reported values using traditional flow-FISH and Southern blotting. This modification to the traditional flow-FISH technique should therefore allow simultaneous fluorescent immunophenotyping and telomere length measurement, permitting complex cell subset-specific analysis in small numbers of cells without the requirement for prior cell sorting.
Figures
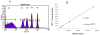


Similar articles
-
Brilliant violet fluorochromes in simultaneous multicolor flow cytometry-fluorescence in situ hybridization measurement of monocyte subsets and telomere length in heart failure.Lab Invest. 2016 Nov;96(11):1223-1230. doi: 10.1038/labinvest.2016.100. Epub 2016 Sep 12. Lab Invest. 2016. PMID: 27617397
-
Simultaneous flow cytometric analysis of two cell surface markers, telomere length, and DNA content.Cytometry. 2002 Nov 1;49(3):96-105. doi: 10.1002/cyto.10163. Cytometry. 2002. PMID: 12442309
-
Telomere length measurement by fluorescence in situ hybridization and flow cytometry: tips and pitfalls.Cytometry. 2002 Feb 1;47(2):89-99. doi: 10.1002/cyto.10053. Cytometry. 2002. PMID: 11813198
-
Flow cytometric measurement of telomere length.Cytometry. 2000 Jun 15;42(3):159-64. doi: 10.1002/1097-0320(20000615)42:3<159::aid-cyto1>3.0.co;2-9. Cytometry. 2000. PMID: 10861688 Review.
-
Measurement of telomere length using PNA probe by cytometry.Methods Cell Biol. 2011;103:189-202. doi: 10.1016/B978-0-12-385493-3.00008-5. Methods Cell Biol. 2011. PMID: 21722804 Review.
Cited by
-
Assessment of telomere length, phenotype, and DNA content.Curr Protoc Cytom. 2004 Sep;Chapter 7:Unit 7.26. doi: 10.1002/0471142956.cy0726s29. Curr Protoc Cytom. 2004. PMID: 18770803 Free PMC article.
-
Interphase Chromosome Flow-FISH.Blood. 2012 Oct 11;120(15):e54-9. doi: 10.1182/blood-2012-05-434266. Epub 2012 Aug 29. Blood. 2012. PMID: 22932794 Free PMC article.
-
Generation of autologous tumor-specific T cells for adoptive transfer based on vaccination, in vitro restimulation and CD3/CD28 dynabead-induced T cell expansion.Cancer Immunol Immunother. 2012 Aug;61(8):1221-31. doi: 10.1007/s00262-011-1199-8. Epub 2012 Jan 12. Cancer Immunol Immunother. 2012. PMID: 22237888 Free PMC article.
-
Multifunctional cytomegalovirus (CMV)-specific CD8(+) T cells are not restricted by telomere-related senescence in young or old adults.Immunology. 2015 Apr;144(4):549-60. doi: 10.1111/imm.12409. Immunology. 2015. PMID: 25314332 Free PMC article.
-
Brilliant violet fluorochromes in simultaneous multicolor flow cytometry-fluorescence in situ hybridization measurement of monocyte subsets and telomere length in heart failure.Lab Invest. 2016 Nov;96(11):1223-1230. doi: 10.1038/labinvest.2016.100. Epub 2016 Sep 12. Lab Invest. 2016. PMID: 27617397
References
-
- Baerlocher GM, Mak J, Lansdorp PM. Telomere length measurement by fluorescence in situ hybridization and flow cytometry: tips and pitfalls. Cytometry A. 2002;47:89. - PubMed
-
- Baerlocher GM, Lansdorp PM. Telomere length measurements in leukocyte subsets by automated multicolor FISH-flow. Cytometry A. 2003;55A:1. - PubMed
-
- Baerlocher GM, Vulto I, de Jong G, Lansdorp PM. Flow cytometry and FISH to measure the average length of telomeres (FISH-flow) Nature Protocols. 2006;1:2365. - PubMed
-
- Blackburn EH. Telomere states and cell fates. Nature. 2000;408:53. - PubMed
-
- Bulte JWM, Modo MMJ. Nanoparticles in Biomedical Imaging. Springer; New York, NY: 2008. Part 5 - Quantum dots: Applications in Optical Imaging; p. 413.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

