Multiple regulatory and effector roles of autophagy in immunity
- PMID: 19269148
- PMCID: PMC2788943
- DOI: 10.1016/j.coi.2009.02.002
Multiple regulatory and effector roles of autophagy in immunity
Abstract
Autophagy is a cytoplasmic homeostasis pathway, enabling cells to digest their own cytosol, remove toxic protein aggregates, and eliminate defective or surplus organelles. A plenitude of studies has now expanded roles of autophagy to both effector and regulatory functions in innate and adaptive immunity. In its role of an immunological effector, autophagy plays many parts: (i) In its most primeval manifestation, it captures and digests intracellular microbes, (ii) it is an antimicrobial output of Toll-like receptor (TLR) response to pathogen associated molecular patterns (PAMP), and (iii) it is an effector of Th1-Th2 polarization in resistance or susceptibility to intracellular pathogens. As a regulator of immunity, autophagy plays a multitude of functions: (i) It acts as a topological inversion device servicing both innate and adaptive immunity by delivering cytosolic antigens to the lumen of MHC II compartments and cytosolic PAMPs to endosomal TLRs, (ii) it is crucial in T cell repertoire selection in the thymus and control of central tolerance, (iii) it plays a role in T and B cell homeostasis, and (iv) it is of significance for inflammatory pathology. A properly functioning autophagy helps prevent autoimmunity and assists in clearing pathogens. When aberrant, it contributes to human inflammatory disorders such as Crohn's disease.
Figures


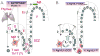
Comment in
-
Innate resistance and inflammation.Curr Opin Immunol. 2009 Feb;21(1):1-2. doi: 10.1016/j.coi.2009.02.001. Epub 2009 Feb 14. Curr Opin Immunol. 2009. PMID: 19223161 No abstract available.
References
-
- Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. Embo J. 2007;26:1749–1760. Shows that reactive oxygen species released from mitochondria induce autophagy by inactivating Atg4, stabilizing lipidated LC3. - PMC - PubMed
-
- Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

