Determination of AMP-activated protein kinase phosphorylation sites in recombinant protein expressed using the pET28a vector: a cautionary tale
- PMID: 19269329
- PMCID: PMC2691924
- DOI: 10.1016/j.pep.2009.02.016
Determination of AMP-activated protein kinase phosphorylation sites in recombinant protein expressed using the pET28a vector: a cautionary tale
Abstract
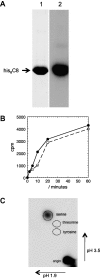
AMP-activated protein kinase (AMPK) is responsible for sensing of the cell's energetic status and it phosphorylates numerous substrates involved in anabolic and catabolic processes as well as interacting with signaling cascades. Mutations in the gene encoding the gamma 2 regulatory subunit have been shown to cause hypertrophic cardiomyopathy (HCM) with conduction abnormalities. As part of a study to examine the role of AMPK in the heart, we tested whether specific domains of the thick filament component cardiac myosin binding protein-C (cMyBP-C) were good in vitro AMPK substrates. The commercially available pET28a expression vector was used to generate a recombinant form of the cMyBP-C C8 domain as a fusion protein with a hexahistidine tag. In vitro phosphorylation with activated kinase showed that the purified fusion protein was a good AMPK substrate, phosphorylated at a similar rate to the control SAMS peptide and with phosphate incorporation specifically in serine residues. However, subsequent analysis of alanine replacement mutants and thrombin digestion revealed that the strong AMPK phosphorylation site was contained within the thrombin cleavage sequence encoded by the vector. As this sequence is common to many commercial pET vectors, caution is advised in the mapping of AMPK phosphorylation sites when this sequence is present.
Figures
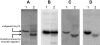
Similar articles
-
Phosphorylation of serine residues in histidine-tag sequences attached to recombinant protein kinases: a cause of heterogeneity in mass and complications in function.Protein Expr Purif. 2005 Dec;44(2):121-9. doi: 10.1016/j.pep.2005.04.018. Protein Expr Purif. 2005. PMID: 15946859
-
Analysis of the specificity of the AMP-activated protein kinase by site-directed mutagenesis of bacterially expressed 3-hydroxy 3-methylglutaryl-CoA reductase, using a single primer variant of the unique-site-elimination method.Eur J Biochem. 1996 May 1;237(3):800-8. doi: 10.1111/j.1432-1033.1996.0800p.x. Eur J Biochem. 1996. PMID: 8647128
-
Protein kinase substrate recognition studied using the recombinant catalytic domain of AMP-activated protein kinase and a model substrate.J Mol Biol. 2002 Mar 22;317(2):309-23. doi: 10.1006/jmbi.2001.5316. J Mol Biol. 2002. PMID: 11902845
-
AMP-activated protein kinase in metabolic control and insulin signaling.Circ Res. 2007 Feb 16;100(3):328-41. doi: 10.1161/01.RES.0000256090.42690.05. Circ Res. 2007. PMID: 17307971 Review.
-
AMP-activated protein kinase: a key regulator of energy balance with many roles in human disease.J Intern Med. 2014 Dec;276(6):543-59. doi: 10.1111/joim.12268. Epub 2014 May 27. J Intern Med. 2014. PMID: 24824502 Free PMC article. Review.
References
-
- Hardie D.G. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat. Rev. 2007;8:774–785. - PubMed
-
- Carling D., Sanders M.J., Woods A. The regulation of AMP-activated protein kinase by upstream kinases. Int. J. Obes. (Lond) 2008;32(Suppl. 4):S55–S59. - PubMed
-
- Blair E., Redwood C., Ashrafian H., Oliveira M., Broxholme J., Kerr B., Salmon A., Ostman-Smith I., Watkins H. Mutations in the gamma(2) subunit of AMP-activated protein kinase cause familial hypertrophic cardiomyopathy: evidence for the central role of energy compromise in disease pathogenesis. Hum. Mol. Genet. 2001;10:1215–1220. - PubMed
-
- Lang T., Yu L., Tu Q., Jiang J., Chen Z., Xin Y., Liu G., Zhao S. Molecular cloning, genomic organization, and mapping of PRKAG2, a heart abundant gamma2 subunit of 5′-AMP-activated protein kinase, to human chromosome 7q36. Genomics. 2000;70:258–263. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

