Fibroblast growth factor 2-stimulated proliferation is lower in muscle precursor cells from old rats
- PMID: 19270036
- PMCID: PMC4821009
- DOI: 10.1113/expphysiol.2008.046136
Fibroblast growth factor 2-stimulated proliferation is lower in muscle precursor cells from old rats
Abstract
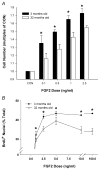
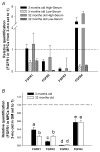
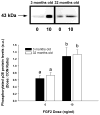
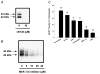
In aged skeletal muscle, impairments in regrowth and regeneration may be explained by a decreased responsiveness of muscle precursor cells (MPCs) to environmental cues such as growth factors. We hypothesized that impaired responsiveness to fibroblast growth factor 2 (FGF2) in MPCs from old animals would be explained by impaired FGF2 signalling. We determined that 5-bromo-2'-deoxyuridine (BrdU) incorporation and cell number increase less in MPCs from 32- compared with 3-month-old rats. In the presence of FGF2, we demonstrated that there were age-associated differential expression patterns for FGF receptor 1 and 2 mRNAs. Measurement of downstream signalling revealed that that mitogen-activated protein kinase/ERK kinase 1/2 (MEK1/2)-extracellular signal-regulated kinase 1/2, protein kinase C and p38 were FGF2-driven pathways in MPCs. Uniquely, protein kinase C signalling was shown to play the largest role in FGF2-stimulated proliferation in MPCs. c-Jun N-terminal kinase (JNK) signalling was ruled out as an FGF2-stimulated proliferation pathway in MPCs. Inhibition of JNK had no effect on FGF2 signalling to BrdU incorporation, and FGF2 treatment was associated with increased phosphorylation of p38, which inhibits, rather than stimulates, BrdU incorporation in MPCs. Surprisingly, the commonly used vehicle, dimethyl sulphoxide, rescued proliferation in MPCs from old animals. These findings provide insight for the development of effective treatment strategies that target the age-related impairments of MPC proliferation in old skeletal muscle.
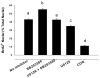
Figures


References
-
- Allen RE, Boxhorn LK. Regulation of skeletal muscle satellite cell proliferation and differentiation by transforming growth factor-β, insulin-like growth factor I, and fibroblast growth factor. J Cell Physiol. 1989;138:311–315. - PubMed
-
- Allen RE, Temm-Grove CJ, Sheehan SM, Rice G. Skeletal muscle satellite cell cultures. Methods Cell Biol. 1997;52:155–176. - PubMed
-
- Anderson JE, Mitchell CM, McGeachie JK, Grounds MD. The time course of basic fibroblast growth factor expression in crush-injured skeletal muscles of SJL/J and BALB/c mice. Exp Cell Res. 1995;216:325–334. - PubMed
-
- Brien S, Prescott P, Bashir N, Lewith H, Lewith G. Systematic review of the nutritional supplements dimethyl sulfoxide (DMSO) and methylsulfonylmethane (MSM) in the treatment of osteoarthritis. Osteoarthritis Cartilage. 2008;16:1277–1288. - PubMed
-
- Brooks SV, Faulkner JA. Contraction-induced injury: recovery of skeletal muscles in young and old mice. Am J Physiol Cell Physiol. 1990;258:C436–C442. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

