Postthymic maturation influences the CD8 T cell response to antigen
- PMID: 19270077
- PMCID: PMC2660772
- DOI: 10.1073/pnas.0812354106
Postthymic maturation influences the CD8 T cell response to antigen
Abstract
Complete T cell development requires postthymic maturation, and we investigated the influence of this ontological period on the CD8 T cell response to infection by comparing responses of mature CD8 T cells with those of recent thymic emigrants (RTEs). When activated with a noninflammatory stimulus or a bacterial or viral pathogen, CD8 RTEs generated a lower proportion of cytokine-producing effector cells and long-lived memory precursors compared with their mature counterparts. Although peripheral T cell maturation is complete within several weeks after thymic egress, RTE-derived memory cells continued to express inappropriate levels of memory cell markers and display an altered pattern of cytokine production, even 8 weeks after infection. When rechallenged, RTE-derived memory cells generated secondary effector cells that were phenotypically and functionally equivalent to those generated by their mature counterparts. The defects at the effector and memory stages were not associated with differences in the expression of T cell receptor-, costimulation-, or activation-associated cell surface markers yet were associated with lower Ly6C expression levels at the effector stage. This work demonstrates that the stage of postthymic maturation influences cell fate decisions and cytokine profiles of stimulated CD8 T cells, with repercussions that are apparent long after cells have progressed from the RTE compartment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

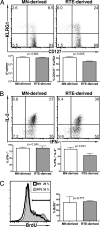

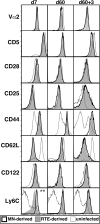
References
-
- Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–192. - PubMed
-
- Harty JT, Badovinac VP. Shaping and reshaping CD8+ T cell memory. Nat Rev Immunol. 2008;8:107–119. - PubMed
-
- Joshi NS, Kaech SM. Effector CD8 T cell development: A balancing act between memory cell potential and terminal differentiation. J Immunol. 2008;180:1309–1315. - PubMed
-
- Saparov A, et al. Interleukin-2 expression by a subpopulation of primary T cells is linked to enhanced memory/effector function. Immunity. 1999;11:271–280. - PubMed
-
- Yu W, et al. Continued RAG expression in late stages of B cell development and no apparent reinduction after immunization. Nature. 1999;400:682–687. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

