Biosynthesis of sibiromycin, a potent antitumor antibiotic
- PMID: 19270142
- PMCID: PMC2681668
- DOI: 10.1128/AEM.02326-08
Biosynthesis of sibiromycin, a potent antitumor antibiotic
Abstract
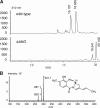
Pyrrolobenzodiazepines, a class of natural products produced by actinomycetes, are sequence selective DNA alkylating compounds with significant antitumor properties. Among the pyrrolo[1,4]benzodiazepines (PBDs) sibiromycin, one of two identified glycosylated PBDs, displays the highest affinity for DNA and the most potent antitumor properties. Despite the promising antitumor properties clinical trials of sibiromycin were precluded by the cardiotoxicity effect in animals attributed to the presence of the C-9 hydroxyl group. As a first step toward the development of sibiromycin analogs, we have cloned and localized the sibiromycin gene cluster to a 32.7-kb contiguous DNA region. Cluster boundaries tentatively assigned by comparative genomics were verified by gene replacement experiments. The sibiromycin gene cluster consisting of 26 open reading frames reveals a "modular" strategy in which the synthesis of the anthranilic and dihydropyrrole moieties is completed before assembly by the nonribosomal peptide synthetase enzymes. In addition, the gene cluster identified includes open reading frames encoding enzymes involved in sibirosamine biosynthesis, as well as regulatory and resistance proteins. Gene replacement and chemical complementation studies are reported to support the proposed biosynthetic pathway.
Figures




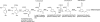
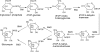
References
-
- Antonow, D., N. Cooper, P. W. Howard, and D. E. Thurston. 2007. Parallel synthesis of a novel C2-aryl pyrrolo[2,1-c][1,4]benzodiazepine (PBD) library. J. Comb. Chem. 9:437-445. - PubMed
-
- Brahme, N. M., J. E. Gonzalez, J. P. Rolls, E. J. Hessler, S. Mizsak, and L. H. Hurley. 1984. Biosynthesis of the lincomycins. 1. Studies using stable isotopes on the biosynthesis of the propyl-l-hygric and ethyl-l-hygric acid moieties of lincomycin-A and lincomycin-B. J. Am. Chem. Soc. 106:7873-7878.
-
- Cargill, C., E. Bachmann, and G. Zbinden. 1974. Effects of daunomycin and anthramycin on electrocardiogram and mitochondrial metabolism of the rat heart. J. Natl. Cancer Inst. 53:481-486. - PubMed
-
- Clingen, P. H., I. U. De Silva, P. J. McHugh, F. J. Ghadessy, M. J. Tilby, D. E. Thurston, and J. A. Hartley. 2005. The XPF-ERCC1 endonuclease and homologous recombination contribute to the repair of minor groove DNA interstrand crosslinks in mammalian cells produced by the pyrrolo[2,1-c][1,4]benzodiazepine dimer SJG-136. Nucleic Acids Res. 33:3283-3291. - PMC - PubMed
-
- Coutinho, P. M., E. Deleury, G. J. Davies, and B. Henrissat. 2003. An evolving hierarchical family classification for glycosyltransferases. J. Mol. Biol. 328:307-317. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

