Cloning and characterization of the biosynthetic gene cluster for tomaymycin, an SJG-136 monomeric analog
- PMID: 19270147
- PMCID: PMC2681672
- DOI: 10.1128/AEM.02325-08
Cloning and characterization of the biosynthetic gene cluster for tomaymycin, an SJG-136 monomeric analog
Abstract
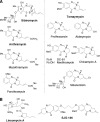
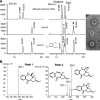
Tomaymycin produced by Streptomyces achromogenes is a naturally produced pyrrolobenzodiazepine (PBD). The biosynthetic gene cluster for tomaymycin was identified and sequenced. The gene cluster analysis reveals a novel biosynthetic pathway for the anthranilate moiety of PBDs. Gene replacement and chemical complementation studies were used to confirm the proposed biosynthetic pathway.
Figures


Similar articles
-
Draft genome sequence of marine Streptomyces sp. strain W007, which produces angucyclinone antibiotics with a benz[a]anthracene skeleton.J Bacteriol. 2012 Mar;194(6):1628-9. doi: 10.1128/JB.06701-11. J Bacteriol. 2012. PMID: 22374958 Free PMC article.
-
Identification of the herboxidiene biosynthetic gene cluster in Streptomyces chromofuscus ATCC 49982.Appl Environ Microbiol. 2012 Mar;78(6):2034-8. doi: 10.1128/AEM.06904-11. Epub 2012 Jan 13. Appl Environ Microbiol. 2012. PMID: 22247174 Free PMC article.
-
Identification and analysis of the biosynthetic gene cluster encoding the thiopeptide antibiotic cyclothiazomycin in Streptomyces hygroscopicus 10-22.Appl Environ Microbiol. 2010 Apr;76(7):2335-44. doi: 10.1128/AEM.01790-09. Epub 2010 Feb 12. Appl Environ Microbiol. 2010. PMID: 20154110 Free PMC article.
-
Insights into the evolution of macrolactam biosynthesis through cloning and comparative analysis of the biosynthetic gene cluster for a novel macrocyclic lactam, ML-449.Appl Environ Microbiol. 2010 Jan;76(1):283-93. doi: 10.1128/AEM.00744-09. Epub 2009 Oct 23. Appl Environ Microbiol. 2010. PMID: 19854930 Free PMC article.
-
Cloning and characterization of the polyether salinomycin biosynthesis gene cluster of Streptomyces albus XM211.Appl Environ Microbiol. 2012 Feb;78(4):994-1003. doi: 10.1128/AEM.06701-11. Epub 2011 Dec 9. Appl Environ Microbiol. 2012. PMID: 22156425 Free PMC article.
Cited by
-
Comparative Genomics and Biosynthetic Potential Analysis of Two Lichen-Isolated Amycolatopsis Strains.Front Microbiol. 2018 Mar 13;9:369. doi: 10.3389/fmicb.2018.00369. eCollection 2018. Front Microbiol. 2018. PMID: 29593664 Free PMC article.
-
Natural products from thioester reductase containing biosynthetic pathways.Nat Prod Rep. 2018 Sep 19;35(9):847-878. doi: 10.1039/c8np00013a. Nat Prod Rep. 2018. PMID: 29916519 Free PMC article. Review.
-
Biosynthesis of sibiromycin, a potent antitumor antibiotic.Appl Environ Microbiol. 2009 May;75(9):2869-78. doi: 10.1128/AEM.02326-08. Epub 2009 Mar 6. Appl Environ Microbiol. 2009. PMID: 19270142 Free PMC article.
-
Anthranilate-activating modules from fungal nonribosomal peptide assembly lines.Biochemistry. 2010 Apr 20;49(15):3351-65. doi: 10.1021/bi100198y. Biochemistry. 2010. PMID: 20225828 Free PMC article.
-
Crystal Structures of L-DOPA Dioxygenase from Streptomyces sclerotialus.Biochemistry. 2019 Dec 31;58(52):5339-5350. doi: 10.1021/acs.biochem.9b00396. Epub 2019 Jun 25. Biochemistry. 2019. PMID: 31180203 Free PMC article.
References
-
- Alley, M. C., M. G. Hollingshead, C. M. Pacula-Cox, W. R. Waud, J. A. Hartley, P. W. Howard, S. J. Gregson, D. E. Thurston, and E. A. Sausville. 2004. SJG-136 (NSC 694501), a novel rationally designed DNA minor groove interstrand cross-linking agent with potent and broad spectrum antitumor activity. 2. Efficacy evaluations. Cancer Res. 64:6700-6706. - PubMed
-
- Antonow, D., N. Cooper, P. W. Howard, and D. E. Thurston. 2007. Parallel synthesis of a novel C2-aryl pyrrolo[2,1-c][1,4]benzodiazepine (PBD) library. J. Comb. Chem. 9:437-445. - PubMed
-
- Brahme, N. M., J. E. Gonzalez, J. P. Rolls, E. J. Hessler, S. Mizsak, and L. H. Hurley. 1984. Biosynthesis of the lincomycins. 1. Studies using stable isotopes on the biosynthesis of the propyl-l-hygric and ethyl-l-hygric acid moieties of lincomycin-A and lincomycin-B. J. Am. Chem. Soc. 106:7873-7878.
-
- Calisti, C., A. G. Ficca, P. Barghini, and M. Ruzzi. 2008. Regulation of ferulic catabolic genes in Pseudomonas fluorescens BF13: involvement of a MarR family regulator. Appl. Microbiol. Biotechnol. 80:475-483. - PubMed
-
- Reference deleted.
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

