Regulated degradation of FANCM in the Fanconi anemia pathway during mitosis
- PMID: 19270156
- PMCID: PMC2658523
- DOI: 10.1101/gad.1761309
Regulated degradation of FANCM in the Fanconi anemia pathway during mitosis
Erratum in
- Genes Dev. 2009 Apr 15;23(8):1025.. D'Andrea, Alan [corrected to D'Andrea, Alan D]
Abstract
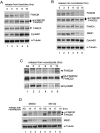
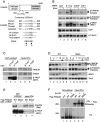
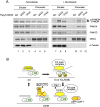
The 13 Fanconi anemia (FA) proteins cooperate in a common DNA repair pathway. Eight of these proteins are assembled into a multisubunit E3 ligase called the FA core complex. During S phase, the FA core complex is loaded by the FANCM protein into chromatin where it monoubiquitinates its substrates. In mitosis, the FA core complex is released from FANCM by an unknown mechanism. Here we show that FANCM is hyperphosphorylated and degraded during mitosis. beta-TRCP and Plk1 are the key regulators of FANCM degradation. Nondegradable mutant forms of FANCM retain the FA core complex in the chromatin and disrupt the FA pathway. Our data provide a novel mechanism for the cell cycle-dependent regulation of the FA pathway.
Figures

References
-
- Branzei D., Foiani M. Regulation of DNA repair throughout the cell cycle. Nat. Rev. Mol. Cell Biol. 2008;9:297–308. - PubMed
-
- Brummelkamp T.R., Bernards R., Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–553. - PubMed
-
- Busino L., Donzelli M., Chiesa M., Guardavaccaro D., Ganoth D., Dorrello N.V., Hershko A., Pagano M., Draetta G.F. Degradation of Cdc25A by β-TrCP during S phase and in response to DNA damage. Nature. 2003;426:87–91. - PubMed
-
- Ciccia A., Ling C., Coulthard R., Yan Z., Xue Y., Meetei A.R., Laghmani el H., Joenje H., McDonald N., de Winter J.P., Wang W., West S.C. Identification of FAAP24, a Fanconi anemia core complex protein that interacts with FANCM. Mol. Cell. 2007;25:331–343. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
