Molecules and mechanisms of dendrite development in Drosophila
- PMID: 19270170
- PMCID: PMC2685926
- DOI: 10.1242/dev.014423
Molecules and mechanisms of dendrite development in Drosophila
Abstract
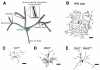
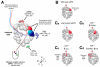
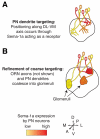
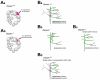
Neurons are one of the most morphologically diverse cell types, in large part owing to their intricate dendrite branching patterns. Dendrites are structures that are specialized to receive and process inputs in neurons, thus their specific morphologies reflect neural connectivity and influence information flow through circuits. Recent studies in Drosophila on the molecular basis of dendrite diversity, dendritic guidance, the cell biology of dendritic branch patterning and territory formation have identified numerous intrinsic and extrinsic cues that shape diverse features of dendrites. As we discuss in this review, many of the mechanisms that are being elucidated show conservation in diverse systems.
Figures


References
-
- Agarwala, K. L., Nakamura, S., Tsutsumi, Y. and Yamakawa, K. (2000). Down syndrome cell adhesion molecule DSCAM mediates homophilic intercellular adhesion. Brain Res. Mol. Brain Res. 79, 118-126. - PubMed
-
- Agarwala, K. L., Ganesh, S., Amano, K., Suzuki, T. and Yamakawa, K. (2001). DSCAM, a highly conserved gene in mammals, expressed in differentiating mouse brain. Biochem. Biophys. Res. Commun. 281, 697-705. - PubMed
-
- Ainsley, J. A., Pettus, J. M., Bosenko, D., Gerstein, C. E., Zinkevich, N., Anderson, M. G., Adams, C. M., Welsh, M. J. and Johnson, W. A. (2003). Enhanced locomotion caused by loss of the Drosophila DEG/ENaC protein Pickpocket1. Curr. Biol. 13, 1557-1563. - PubMed
-
- Aizawa, H., Hu, S. C., Bobb, K., Balakrishnan, K., Ince, G., Gurevich, I., Cowan, M. and Ghosh, A. (2004). Dendrite development regulated by CREST, a calcium-regulated transcriptional activator. Science 303, 197-202. - PubMed
-
- Alvarez, V. A. and Sabatini, B. L. (2007). Anatomical and physiological plasticity of dendritic spines. Annu. Rev. Neurosci. 30, 79-97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

