Glutathione-S-transferase P protects against endothelial dysfunction induced by exposure to tobacco smoke
- PMID: 19270193
- PMCID: PMC2685347
- DOI: 10.1152/ajpheart.00867.2008
Glutathione-S-transferase P protects against endothelial dysfunction induced by exposure to tobacco smoke
Abstract
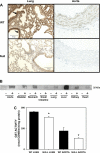
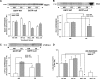
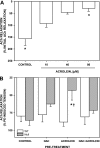
Exposure to tobacco smoke impairs endothelium-dependent arterial dilation. Reactive constituents of cigarette smoke are metabolized and detoxified by glutathione-S-transferases (GSTs). Although polymorphisms in GST genes are associated with the risk of cancer in smokers, the role of these enzymes in regulating the cardiovascular effects of smoking has not been studied. The P isoform of GST (GSTP), which catalyzes the conjugation of electrophilic molecules in cigarette smoke such as acrolein, was expressed in high abundance in the mouse lung and aorta. Exposure to tobacco smoke for 3 days (5 h/day) decreased total plasma protein. These changes were exaggerated in GSTP(-/-) mice. Aortic rings isolated from tobacco smoke-exposed GSTP(-/-) mice showed greater attenuation of ACh-evoked relaxation than those from GSTP(+/+) mice. The lung, plasma, and aorta of mice exposed to tobacco smoke or acrolein (for 5 h) accumulated more acrolein-adducted proteins than those tissues of mice exposed to air, indicating that exposure to tobacco smoke results in the systemic delivery of acrolein. Relative to GSTP(+/+) mice, modification of some proteins by acrolein was increased in the aorta of GSTP(-/-) mice. Aortic rings prepared from GSTP(-/-) mice that inhaled acrolein (1 ppm, 5 h/day for 3 days) or those exposed to acrolein in an organ bath showed diminished ACh-induced arterial relaxation more strongly than GSTP(+/+) mice. Acrolein-induced endothelial dysfunction was prevented by pretreatment of the aorta with N-acetylcysteine. These results indicate that GSTP protects against the endothelial dysfunction induced by tobacco smoke exposure and that this protection may be related to the detoxification of acrolein or other related cigarette smoke constituents.
Figures




References
-
- Awasthi YC, Sharma R, Singhal SS. Human glutathione S-transferases. Int J Biochem 26: 295–308, 1994. - PubMed
-
- Awasthi YC, Yang Y, Tiwari NK, Patrick B, Sharma A, Li J, Awasthi S. Regulation of 4-hydroxynonenal-mediated signaling by glutathione S-transferases. Free Radic Biol Med 37: 607–619, 2004. - PubMed
-
- Barnoya J, Glantz SA. Cardiovascular effects of secondhand smoke: nearly as large as smoking. Circulation 111: 2684–2698, 2005. - PubMed
-
- Barua RS, Ambrose JA, Eales-Reynolds LJ, DeVoe MC, Zervas JG, Saha DC. Dysfunctional endothelial nitric oxide biosynthesis in healthy smokers with impaired endothelium-dependent vasodilatation. Circulation 104: 1905–1910, 2001. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

