Overview of therapeutic drug monitoring
- PMID: 19270474
- PMCID: PMC2687654
- DOI: 10.3904/kjim.2009.24.1.1
Overview of therapeutic drug monitoring
Abstract
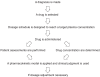
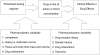
Therapeutic drug monitoring (TDM) is the clinical practice of measuring specific drugs at designated intervals to maintain a constant concentration in a patient's bloodstream, thereby optimizing individual dosage regimens. It is unnecessary to employ TDM for the majority of medications, and it is used mainly for monitoring drugs with narrow therapeutic ranges, drugs with marked pharmacokinetic variability, medications for which target concentrations are difficult to monitor, and drugs known to cause therapeutic and adverse effects. The process of TDM is predicated on the assumption that there is a definable relationship between dose and plasma or blood drug concentration, and between concentration and therapeutic effects. TDM begins when the drug is first prescribed, and involves determining an initial dosage regimen appropriate for the clinical condition and such patient characteristics as age, weight, organ function, and concomitant drug therapy. When interpreting concentration measurements, factors that need to be considered include the sampling time in relation to drug dose, dosage history, patient response, and the desired medicinal targets. The goal of TDM is to use appropriate concentrations of difficult-to-manage medications to optimize clinical outcomes in patients in various clinical situations.
Figures


References
-
- Touw DJ, Neef C, Thomson AH, Vinks AA. Cost-effectiveness of therapeutic drug monitoring: a systemic review. Ther Drug Monit. 2005;27:10–17. - PubMed
-
- Birkett DJ. Pharmacokinetics made easy: therapeutic drug monitoring. Aust Prescr. 1997;20:9–11.
-
- Tange SM, Grey VL, Senecal PE. Therapeutic drug monitoring in pediatrics: a need for improvement. J Clin Pharmacol. 1994;34:200–214. - PubMed
-
- Reed MD, Blumer JL. Therapeutic drug monitoring in the pediatric intensive care unit. Pediatr Clin North Am. 1994;41:1227–1243. - PubMed
-
- Kearns GL, Moss MM, Clayton BD, Hewett DD. Pharmacokinetics and efficacy of digoxin specific Fab fragments in a child following massive digoxin overdose. J Clin Pharmacol. 1989;29:901–908. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
