Hypoxia alters cell cycle regulatory protein expression and induces premature maturation of oligodendrocyte precursor cells
- PMID: 19270736
- PMCID: PMC2649433
- DOI: 10.1371/journal.pone.0004739
Hypoxia alters cell cycle regulatory protein expression and induces premature maturation of oligodendrocyte precursor cells
Abstract
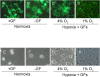
Background: Periventricular white matter injury (PWMI) is a common form of brain injury sustained by preterm infants. A major factor that predisposes to PWMI is hypoxia. Because oligodendrocytes (OLs) are responsible for myelination of axons, abnormal OL development or function may affect brain myelination. At present our understanding of the influences of hypoxia on OL development is limited. To examine isolated effects of hypoxia on OLs, we examined the influences of hypoxia on OL development in vitro.
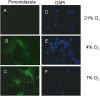
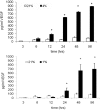
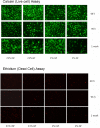
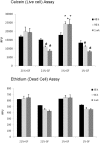
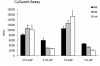
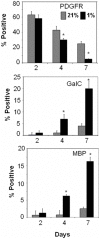
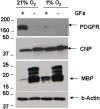
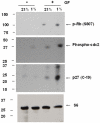
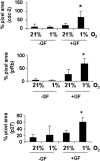
Methodology/findings: Cultures of oligodendrocyte precursor cells (OPCs) were prepared from mixed glial cultures and were 99% pure. OPCs were maintained at 21% O(2) or hypoxia (1% or 4% O(2)) for up to 7 days. We observed that 1% O(2) lead to an increase in the proportion of myelin basic protein (MBP)-positive OLs after 1 week in culture, and a decrease in the proportion of platelet-derived growth factor receptor alpha (PDGFRalpha)-positive cells suggesting premature OL maturation. Increased expression of the cell cycle regulatory proteins p27(Kip1) and phospho-cdc2, which play a role in OL differentiation, was seen as well.
Conclusions: These results show that hypoxia interferes with the normal process of OL differentiation by inducing premature OPC maturation.
Conflict of interest statement
Figures


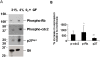
References
-
- Rezaie P, Dean A. Periventricular leukomalacia, inflammation and white matter lesions within the developing nervous system. Neuropathology. 2002;22:106–132. - PubMed
-
- Ment LR, Schwartz M, Makuch RW, Stewart WB. Association of chronic sublethal hypoxia with ventriculomegaly in the developing rat brain. Brain ResearchDevelopmentalBrain Research. 1998;111:197–203. - PubMed
-
- Back SA, Volpe JJ. Cellular and molecular pathogenesis of periventricular white matter injury. MRDD Res Rev. 1997;3:96–107.
-
- Back SA. Approaches to the cellular and molecular pathogenesis of human perinatal white matter injury. In: Choi D, Dacey R Jr, Hsu CH, Powers W, editors. Cerebrovascular disease: Momentum at the end of the second millenium (21st Princeton Conference) Armonk, New York: Futura Press; 2001. pp. 131–160.
-
- Pfeiffer SE, Warrington AE, Bansal R. The oligodendrocyte and its many cellular processes. Trends Cell Biol. 1993;3:191–197. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

