Aggregation of copper-zinc superoxide dismutase in familial and sporadic ALS
- PMID: 19271992
- PMCID: PMC2842589
- DOI: 10.1089/ars.2009.2536
Aggregation of copper-zinc superoxide dismutase in familial and sporadic ALS
Abstract
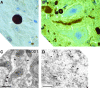
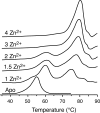
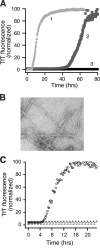
Amyotrophic lateral sclerosis (ALS) is a progressive, fatal neurodegenerative disease characterized by the selective death of motor neurons. While the most common form of ALS is sporadic and has no known cause, a small subset of cases is familial because of underlying genetic mutations. The best-studies example of familial ALS is that caused by mutations in the protein copper-zinc superoxide dismutase. The formation of SOD1-rich inclusions in the spinal cord is an early and prominent feature of SOD1-linked familial ALS in human patients and animal models of this disease. These inclusions have been shown to consist of SOD1-rich fibrils, suggesting that the conversion of soluble SOD1 into amyloid fibrils may play an important role in the etiology of familial ALS. SOD1 is also present in inclusions found in spinal cords of sporadic ALS patients, allowing speculations to arise regarding a possible involvement of SOD1 in the sporadic form of this disease. We here review the recent research on the significance, causes, and mechanisms of SOD1 fibril formation from a biophysical perspective.
Figures


References
-
- Arnesano F. Banci L. Bertini I. Martinelli M. Furukawa Y. O'Halloran TV. The unusually stable quaternary structure of human Cu, Zn-superoxide dismutase 1 is controlled by both metal occupancy and disulfide status. J Biol Chem. 2004;279:47998–48003. - PubMed
-
- Assfalg M. Banci L. Bertini I. Turano P. Vasos PR. Superoxide dismutase folding/unfolding pathway: role of the metal Ions in modulating structural and dynamical features. J Mol Biol. 2003;330:145–158. - PubMed
-
- Banci L. Bertini I. Cantini F. D'Amelio N. Gaggelli E. Human SOD1 before harboring the catalytic metal: Solution structure of copper depleted, disulfide-reduced form. J Biol Chem. 2006;281:2333–2337. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

