Treatment with LL-37 peptide enhances antitumor effects induced by CpG oligodeoxynucleotides against ovarian cancer
- PMID: 19272013
- PMCID: PMC2855250
- DOI: 10.1089/hum.2008.124
Treatment with LL-37 peptide enhances antitumor effects induced by CpG oligodeoxynucleotides against ovarian cancer
Abstract
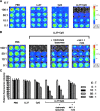
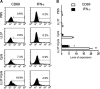
There is an urgent need for innovative therapies against ovarian cancer, one of the leading causes of death from gynecological cancers in the United States. Immunotherapy employing Toll-like receptor (TLR) ligands, such as CpG oligodeoxynucleotides (CpG-ODN), may serve as a potentially promising approach in the control of ovarian tumors. The CpG-ODN requires intracellular delivery into the endosomal compartment, where it can bind to TLR9 in order to activate the immune system. In the current study, we aim to investigate whether the antimicrobial polypeptide from the cathelicidin family, LL-37, could enhance the immunostimulatory effects of CpG-ODN by increasing the uptake of CpG-ODN into the immune cells, thus enhancing the antitumor effects against ovarian cancer. We found that treatment with the combination of CpG-ODN and LL-37 generated significantly better therapeutic antitumor effects and enhanced survival in murine ovarian tumor-bearing mice compared with treatment with CpG-ODN or LL-37 alone. We also observed that treatment with the combination of CpG-ODN and LL-37 enhanced proliferation and activation of natural killer (NK) cells, but not CD4(+) or CD8(+) T cells, in the peritoneal cavity. Furthermore, in vivo antibody depletion experiments indicated that peritoneal NK cells played a critical role in the observed antitumor effects. Thus, our data suggest that the combination of CpG-ODN with LL-37 peptide may lead to the control of ovarian tumors through the activation of innate immunity.
Figures


Similar articles
-
G3139 and other CpG-containing immunostimulatory phosphorothioate oligodeoxynucleotides are potent suppressors of the growth of human tumor xenografts in nude mice.Oligonucleotides. 2006 Spring;16(1):83-93. doi: 10.1089/oli.2006.16.83. Oligonucleotides. 2006. PMID: 16584297
-
CpG oligodeoxynucleotides potentiate the antitumor activity of anti-BST2 antibody.Cancer Sci. 2015 Oct;106(10):1474-8. doi: 10.1111/cas.12738. Cancer Sci. 2015. PMID: 26498112 Free PMC article.
-
Encapsulation in liposomal nanoparticles enhances the immunostimulatory, adjuvant and anti-tumor activity of subcutaneously administered CpG ODN.Cancer Immunol Immunother. 2007 Aug;56(8):1251-64. doi: 10.1007/s00262-006-0276-x. Epub 2007 Jan 23. Cancer Immunol Immunother. 2007. PMID: 17242927 Free PMC article.
-
Structure-dependent immunostimulatory effect of CpG oligodeoxynucleotides and their delivery system.Int J Nanomedicine. 2012;7:2181-95. doi: 10.2147/IJN.S30197. Epub 2012 Apr 27. Int J Nanomedicine. 2012. PMID: 22619554 Free PMC article. Review.
-
The immunobiology and clinical potential of immunostimulatory CpG oligodeoxynucleotides.J Leukoc Biol. 2000 Oct;68(4):455-63. J Leukoc Biol. 2000. PMID: 11037965 Review.
Cited by
-
The immunology of host defence peptides: beyond antimicrobial activity.Nat Rev Immunol. 2016 May;16(5):321-34. doi: 10.1038/nri.2016.29. Epub 2016 Apr 18. Nat Rev Immunol. 2016. PMID: 27087664 Review.
-
LL-37 as a Powerful Molecular Tool for Boosting the Performance of Ex Vivo-Produced Human Dendritic Cells for Cancer Immunotherapy.Pharmaceutics. 2022 Dec 8;14(12):2747. doi: 10.3390/pharmaceutics14122747. Pharmaceutics. 2022. PMID: 36559241 Free PMC article.
-
Plasmacytoid dendritic cells and their therapeutic activity in cancer.Oncoimmunology. 2012 Aug 1;1(5):726-734. doi: 10.4161/onci.20171. Oncoimmunology. 2012. PMID: 22934264 Free PMC article.
-
Recent insights in nanotechnology-based drugs and formulations designed for effective anti-cancer therapy.J Nanobiotechnology. 2016 May 26;14(1):39. doi: 10.1186/s12951-016-0193-x. J Nanobiotechnology. 2016. PMID: 27229857 Free PMC article. Review.
-
Cathelicidins Modulate TLR-Activation and Inflammation.Front Immunol. 2020 Jun 9;11:1137. doi: 10.3389/fimmu.2020.01137. eCollection 2020. Front Immunol. 2020. PMID: 32582207 Free PMC article. Review.
References
-
- Aderem A. Ulevitch R.J. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. - PubMed
-
- Akira S. Takeda K. Toll-like receptor signalling. Nat. Rev. 2004;4:499–511. - PubMed
-
- Akira S. Takeda K. Kaisho T. Toll-like receptors: Critical proteins linking innate and acquired immunity. Nat. Immunol. 2001;2:675–680. - PubMed
-
- Ballas Z.K. Modulation of NK cell activity by CpG oligodeoxynucleotides. Immunologic Res. 2007;39:15–21. - PubMed
-
- Ballas Z.K. Rasmussen W.L. Krieg A.M. Induction of NK activity in murine and human cells by CpG motifs in oligodeoxynucleotides and bacterial DNA. J. Immunol. 1996;157:1840–1845. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

