Neuroprotective mechanisms of puerarin in middle cerebral artery occlusion-induced brain infarction in rats
- PMID: 19272172
- PMCID: PMC2653511
- DOI: 10.1186/1423-0127-16-9
Neuroprotective mechanisms of puerarin in middle cerebral artery occlusion-induced brain infarction in rats
Abstract
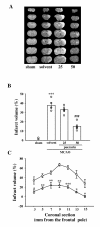
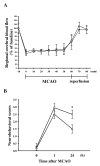
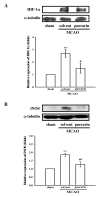
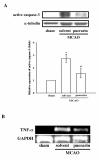
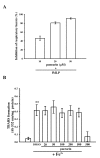
Puerarin, a major isoflavonoid derived from the Chinese medical herb Radix puerariae (kudzu root), has been reported to be useful in the treatment of various cardiovascular diseases. In the present study, we examined the detailed mechanisms underlying the inhibitory effects of puerarin on inflammatory and apoptotic responses induced by middle cerebral artery occlusion (MCAO) in rats. Treatment of puerarin (25 and 50 mg/kg; intraperitoneally) 10 min before MCAO dose-dependently attenuated focal cerebral ischemia in rats. Administration of puerarin at 50 mg/kg, showed marked reduction in infarct size compared with that of control rats. MCAO-induced focal cerebral ischemia was associated with increases in hypoxia-inducible factor-1alpha (HIF-1alpha), inducible nitric oxide synthase (iNOS), and active caspase-3 protein expressions as well as the mRNA expression of tumor necrosis factor-alpha (TNF-alpha) in ischemic regions. These expressions were markedly inhibited by the treatment of puerarin (50 mg/kg). In addition, puerarin (10-50 microM) concentration-dependently inhibited respiratory bursts in human neutrophils stimulated by formyl-Met-Leu-Phe. On the other hand, puerarin (20-500 microM) did not significantly inhibit the thiobarbituric acid-reactive substance reaction in rat brain homogenates. An electron spin resonance (ESR) method was conducted on the scavenging activity of puerarin on the free radicals formed. Puerarin (200 and 500 microM) did not reduce the ESR signal intensity of hydroxyl radical formation. In conclusion, we demonstrate that puerarin is a potent neuroprotective agent on MCAO-induced focal cerebral ischemia in vivo. This effect may be mediated, at least in part, by the inhibition of both HIF-1alpha and TNF-alpha activation, followed by the inhibition of inflammatory responses (i.e., iNOS expression), apoptosis formation (active caspase-3), and neutrophil activation, resulting in a reduction in the infarct volume in ischemia-reperfusion brain injury. Thus, puerarin treatment may represent a novel approach to lowering the risk of or improving function in ischemia-reperfusion brain injury-related disorders.
Figures
Similar articles
-
Nitric Oxide-Dependent Pathways as Critical Factors in the Consequences and Recovery after Brain Ischemic Hypoxia.Biomolecules. 2021 Jul 26;11(8):1097. doi: 10.3390/biom11081097. Biomolecules. 2021. PMID: 34439764 Free PMC article. Review.
-
Inhibitory mechanisms of tetramethylpyrazine in middle cerebral artery occlusion (MCAO)-induced focal cerebral ischemia in rats.Planta Med. 2006 Apr;72(5):411-7. doi: 10.1055/s-2005-917242. Planta Med. 2006. PMID: 16557454
-
Neuroprotective effects of PMC, a potent alpha-tocopherol derivative, in brain ischemia-reperfusion: reduced neutrophil activation and anti-oxidant actions.Biochem Pharmacol. 2007 Mar 1;73(5):682-93. doi: 10.1016/j.bcp.2006.11.009. Epub 2006 Nov 17. Biochem Pharmacol. 2007. PMID: 17157267
-
Neuroprotective effects of xanthohumol, a prenylated flavonoid from hops (Humulus lupulus), in ischemic stroke of rats.J Agric Food Chem. 2012 Feb 29;60(8):1937-44. doi: 10.1021/jf204909p. Epub 2012 Feb 15. J Agric Food Chem. 2012. PMID: 22300539
-
Investigating the Potential Therapeutic Mechanisms of Puerarin in Neurological Diseases.Mol Neurobiol. 2024 Dec;61(12):10747-10769. doi: 10.1007/s12035-024-04222-4. Epub 2024 May 23. Mol Neurobiol. 2024. PMID: 38780722 Review.
Cited by
-
Centella asiatica attenuates the neurobehavioral, neurochemical and histological changes in transient focal middle cerebral artery occlusion rats.Neurol Sci. 2013 Jun;34(6):925-33. doi: 10.1007/s10072-012-1163-1. Epub 2012 Aug 4. Neurol Sci. 2013. PMID: 22864972
-
Neuroprotective effects of total saponins from Rubus parvifolius L. on cerebral ischemia/reperfusion injury in rats.Neural Regen Res. 2012 Jan 25;7(3):176-81. doi: 10.3969/j.issn.1673-5374.2012.03.003. Neural Regen Res. 2012. PMID: 25767495 Free PMC article.
-
Puerarin exhibits greater distribution and longer retention time in neurons than astrocytes in a co-cultured system.Neural Regen Res. 2015 Apr;10(4):605-9. doi: 10.4103/1673-5374.155435. Neural Regen Res. 2015. PMID: 26170822 Free PMC article.
-
Effect of puerarin on transcriptome of astrocyte during oxygen-glucose deprivation/reoxygenation injury.Mol Cell Biochem. 2017 Jan;425(1-2):113-123. doi: 10.1007/s11010-016-2867-y. Epub 2016 Nov 14. Mol Cell Biochem. 2017. PMID: 27844252
-
Nitric Oxide-Dependent Pathways as Critical Factors in the Consequences and Recovery after Brain Ischemic Hypoxia.Biomolecules. 2021 Jul 26;11(8):1097. doi: 10.3390/biom11081097. Biomolecules. 2021. PMID: 34439764 Free PMC article. Review.
References
-
- Chen J, Xu J, Li J. Effect of puerarin on fibrinolytic activity and lipid peroxide in patients with coronary heart disease. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1999;19:649–650. - PubMed
-
- Chen L, Chai Q, Zhao A, Chai X. Effect of puerarin on cerebral blood flow in dogs. Chin Material Med J. 1995;20:560–562. - PubMed
-
- Wang L, Zhao A, Wang F, Chai Q, Chai X. Protective effect of puerarin on acute cerebral ischemia in rats. Chin Material Med J. 1997;22:752–754. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

