Sex differences in learning processes of classical and operant conditioning
- PMID: 19272397
- PMCID: PMC2699937
- DOI: 10.1016/j.physbeh.2009.02.035
Sex differences in learning processes of classical and operant conditioning
Abstract
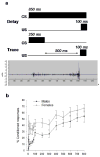
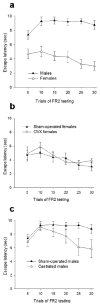
Males and females learn and remember differently at different times in their lives. These differences occur in most species, from invertebrates to humans. We review here sex differences as they occur in laboratory rodent species. We focus on classical and operant conditioning paradigms, including classical eyeblink conditioning, fear-conditioning, active avoidance and conditioned taste aversion. Sex differences have been reported during acquisition, retention and extinction in most of these paradigms. In general, females perform better than males in the classical eyeblink conditioning, in fear-potentiated startle and in most operant conditioning tasks, such as the active avoidance test. However, in the classical fear-conditioning paradigm, in certain lever-pressing paradigms and in the conditioned taste aversion, males outperform females or are more resistant to extinction. Most sex differences in conditioning are dependent on organizational effects of gonadal hormones during early development of the brain, in addition to modulation by activational effects during puberty and adulthood. Critically, sex differences in performance account for some of the reported effects on learning and these are discussed throughout the review. Because so many mental disorders are more prevalent in one sex than the other, it is important to consider sex differences in learning when applying animal models of learning for these disorders. Finally, we discuss how sex differences in learning continue to alter the brain throughout the lifespan. Thus, sex differences in learning are not only mediated by sex differences in the brain, but also contribute to them.
Figures


References
-
- Astur RS, Ortiz ML, Sutherland RJ. A characterization of performance by men and women in a virtual Morris water task: a large and reliable sex difference. Behav Brain Res. 1998;93(12):185–90. - PubMed
-
- Kimura . Sex and Cognition. MA: MIT Press: Cambridge; 1999.
-
- Ullman MT, Miranda RA, Travers ML, editors. Sex differences in the brain, from genes to behavior. Oxford, University Press; 2008. Sex differences in the neurocognition of language; pp. 227–251.
-
- Collins DW, Kimura D. A large sex difference on a two-dimensional mental rotation task. Behav Neurosci. 1997;111(4):845–9. - PubMed
-
- Spence KW, Spence JT. Sex and anxiety differences in eyelid conditioning. Psychol Bull. 1966;65(3):137–42. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

