Inhibition of gelatinase activity reduces neural injury in an ex vivo model of hypoxia-ischemia
- PMID: 19272421
- PMCID: PMC2746919
- DOI: 10.1016/j.neuroscience.2009.02.080
Inhibition of gelatinase activity reduces neural injury in an ex vivo model of hypoxia-ischemia
Abstract
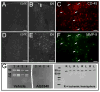
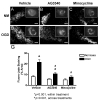
Perinatal hypoxia-ischemia (H-I) often manifests as cognitive and/or motor disturbances that appear early in development. Growing evidence indicates that neuroinflammation may exacerbate H-I injury. Resident microglia release proinflammatory cytokines and proteases in response to ischemia. Matrix metalloproteinases (MMPs), in particular, activate cytokines and degrade basement membrane proteins. These actions ultimately permit entry of peripheral leukocytes into the CNS neuropil, enhancing neuroinflammation and cell death. Currently, the relative contributions of resident and peripheral immune cells to ischemic brain injury are unclear. The present study employed an ex vivo model of H-I through oxygen glucose deprivation (OGD) to identify the cellular localization of MMP-9 in organotypic hippocampal slices from rat, and to determine whether inhibiting gelatin-degrading MMPs affords neuroprotection in the absence of peripheral immune cells. Immunohistochemistry revealed ubiquitous neuronal MMP-9 expression in both normoxic and hypoxic slices. Increased MMP-9 expression was detected in CD11b-positive microglia after 48 h exposure to OGD relative to normoxic controls. Consistent with these data, in situ zymography showed increased gelatinolytic activity after OGD. Gelatin-cleaved fluorescence localized to astrocytic processes and somata of various cellular morphologies. Treatment with either the MMP inhibitor AG3340 (prinomastat) or minocycline dampened OGD-induced gelatinolytic activity and neural injury, as measured by Fluoro-Jade staining, relative to vehicle controls. These results show that resident microglia, in the absence of peripheral immune cells, were sufficient to enhance neural injury after OGD in the organotypic hippocampal slice. Additionally, these effects were associated with upregulation or secretion of MMP-9, and were blocked after treatment with either the gelatinase-selective compound AG3340 or the anti-inflammatory compound minocycline. These data, coupled with the effectiveness of these compounds previously shown in vivo, support the selective targeting of gelatin-degrading MMPs and activated microglia as potential therapeutic approaches to combat neonatal H-I injury.
Figures




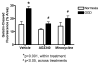
Similar articles
-
Delayed administration of a matrix metalloproteinase inhibitor limits progressive brain injury after hypoxia-ischemia in the neonatal rat.J Neuroinflammation. 2008 Aug 11;5:34. doi: 10.1186/1742-2094-5-34. J Neuroinflammation. 2008. PMID: 18694515 Free PMC article.
-
Minocycline attenuates hypoxia-ischemia-induced neurological dysfunction and brain injury in the juvenile rat.Eur J Neurosci. 2006 Jul;24(2):341-50. doi: 10.1111/j.1460-9568.2006.04918.x. Epub 2006 Jul 12. Eur J Neurosci. 2006. PMID: 16836639
-
Minocycline alleviates hypoxic-ischemic injury to developing oligodendrocytes in the neonatal rat brain.Neuroscience. 2006;137(2):425-35. doi: 10.1016/j.neuroscience.2005.09.023. Epub 2005 Nov 14. Neuroscience. 2006. PMID: 16289838
-
EUK-207, a superoxide dismutase/catalase mimetic, is neuroprotective against oxygen/glucose deprivation-induced neuronal death in cultured hippocampal slices.Brain Res. 2009 Jan 9;1247:28-37. doi: 10.1016/j.brainres.2008.10.016. Epub 2008 Nov 1. Brain Res. 2009. PMID: 18992729 Free PMC article.
-
Delayed minocycline inhibits ischemia-activated matrix metalloproteinases 2 and 9 after experimental stroke.BMC Neurosci. 2006 Jul 17;7:56. doi: 10.1186/1471-2202-7-56. BMC Neurosci. 2006. PMID: 16846501 Free PMC article.
Cited by
-
Splenic immune cells in experimental neonatal hypoxia-ischemia.Transl Stroke Res. 2013 Apr;4(2):208-19. doi: 10.1007/s12975-012-0239-9. Transl Stroke Res. 2013. PMID: 23626659 Free PMC article.
-
Human umbilical cord blood cell therapy blocks the morphological change and recruitment of CD11b-expressing, isolectin-binding proinflammatory cells after middle cerebral artery occlusion.J Neurosci Res. 2010 May 1;88(6):1213-22. doi: 10.1002/jnr.22306. J Neurosci Res. 2010. PMID: 19998484 Free PMC article.
-
Organotypic Hippocampal Slices as Models for Stroke and Traumatic Brain Injury.Mol Neurobiol. 2016 Aug;53(6):4226-4237. doi: 10.1007/s12035-015-9362-4. Epub 2015 Jul 30. Mol Neurobiol. 2016. PMID: 26223803 Free PMC article. Review.
-
Microglia in neurodegeneration.Nat Neurosci. 2018 Oct;21(10):1359-1369. doi: 10.1038/s41593-018-0242-x. Epub 2018 Sep 26. Nat Neurosci. 2018. PMID: 30258234 Free PMC article. Review.
-
Serum matrix metalloproteinase-9 levels and severity of symptoms in boys with attention deficit hyperactivity disorder ADHD/hyperkinetic disorder HKD.Eur Child Adolesc Psychiatry. 2015 Jan;24(1):55-63. doi: 10.1007/s00787-014-0533-z. Epub 2014 Mar 17. Eur Child Adolesc Psychiatry. 2015. PMID: 24633733 Free PMC article.
References
-
- Alvarez-Diaz A, Hilario E, de Cerio FG, Valls-i-Soler A, Alvarez-Diaz FJ. Hypoxic-ischemic injury in the immature brain--key vascular and cellular players. Neonatology. 2007;92:227–235. - PubMed
-
- Alves F, Borchers U, Padge B, Augustin H, Nebendahl K, Kloppel G, Tietze LF. Inhibitory effect of a matrix metalloproteinase inhibitor on growth and spread of human pancreatic ductal adenocarcinoma evaluated in an orthotopic severe combined immunodeficient (SCID) mouse model. Cancer Lett. 2001;165:161–170. - PubMed
-
- Amantea D, Corasaniti MT, Mercuri NB, Bernardi G, Bagetta G. Brain regional and cellular localization of gelatinase activity in rat that have undergone transient middle cerebral artery occlusion. Neuroscience 2008 - PubMed
-
- Apodaca G, Rutka JT, Bouhana K, Berens ME, Giblin JR, Rosenblum ML, McKerrow JH, Banda MJ. Expression of metalloproteinases and metalloproteinase inhibitors by fetal astrocytes and glioma cells. Cancer Res. 1990;50:2322–2329. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

