Differentiation of nonhuman primate embryonic stem cells along neural lineages
- PMID: 19272521
- PMCID: PMC2749555
- DOI: 10.1016/j.diff.2008.10.014
Differentiation of nonhuman primate embryonic stem cells along neural lineages
Abstract
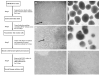
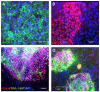
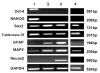
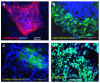
The differentiation of embryonic stem cells (ESCs) into neurons and glial cells represents a promising cell-based therapy for neurodegenerative diseases. Because the rhesus macaque is physiologically and phylogenetically similar to humans, it is a clinically relevant animal model for ESC research. In this study, the pluripotency and neural differentiation potential of a rhesus monkey ESC line (ORMES6) was investigated. ORMES6 was derived from an in vitro produced blastocyst, which is the same way human ESCs have been derived. ORMES6 stably expressed the embryonic transcription factors POU5F1 (Oct4), Sox2 and NANOG. Stage-specific embryonic antigen 4 (SSEA 4) and the glycoproteins TRA-1-60 and TRA-1-81 were also expressed. The embryoid bodies (EBs) formed from ORMES6 ESCs spontaneously gave rise to cells of three germ layers. After exposure to basic fibroblast growth factor (bFGF) for 14-16 days, columnar rosette cells formed in the EB outgrowths. Sox2, microtubule-associated protein (MAP2), beta-tublinIII and glial fibrillary acidic protein (GFAP) genes and Nestin, FoxD3, Pax6 and beta-tublinIII antigens were expressed in the rosette cells. Oct4 and NANOG expression were remarkably down-regulated in these cells. After removal of bFGF from the medium, the rosette cells differentiated along neural lineages. The differentiated cells expressed MAP2, beta-tublinIII, Neuro D and GFAP genes. Most differentiated cells expressed early neuron-specific antigen beta-tublinIII (73+/-4.7%) and some expressed intermediate neuron antigen MAP2 (18+/-7.2%). However, some differentiated cells expressed the glial cell antigens A2B5 (7.17%+/-1.2%), GFAP (4.93+/-1.9%), S100 (7+/-3.5%) and O4 (0.27+/-0.2%). The rosette cells were transplanted into the striatum of immune-deficient NIHIII mice. The cells persisted for approximately 2 weeks and expressed Ki67, NeuN, MAP2 and GFAP. These results demonstrate that the rhesus monkey ESC line ORMES6 retains the pluripotent characteristics of ESCs and can be efficiently induced to differentiate along neural lineages.
Figures


Similar articles
-
Differentiation of monkey embryonic stem cells into neural lineages.Biol Reprod. 2003 May;68(5):1727-35. doi: 10.1095/biolreprod.102.012195. Epub 2002 Dec 11. Biol Reprod. 2003. PMID: 12606331
-
Self-renewal and differentiation capabilities are variable between human embryonic stem cell lines I3, I6 and BG01V.BMC Cell Biol. 2009 Jun 5;10:44. doi: 10.1186/1471-2121-10-44. BMC Cell Biol. 2009. PMID: 19500347 Free PMC article.
-
Human induced pluripotent stem cell-derived neural stem cells survive, migrate, differentiate, and improve neurologic function in a rat model of middle cerebral artery occlusion.Stem Cell Res Ther. 2013 Jun 14;4(3):73. doi: 10.1186/scrt224. Stem Cell Res Ther. 2013. PMID: 23769173 Free PMC article.
-
Engineering the embryoid body microenvironment to direct embryonic stem cell differentiation.Biotechnol Prog. 2009 Jan-Feb;25(1):43-51. doi: 10.1002/btpr.139. Biotechnol Prog. 2009. PMID: 19198003 Free PMC article. Review.
-
Immunogenicity of embryonic stem cell-derived progenitors after transplantation.Curr Opin Organ Transplant. 2011 Feb;16(1):90-5. doi: 10.1097/MOT.0b013e3283424faa. Curr Opin Organ Transplant. 2011. PMID: 21150615 Review.
Cited by
-
Nestin- and doublecortin-positive cells reside in adult spinal cord meninges and participate in injury-induced parenchymal reaction.Stem Cells. 2011 Dec;29(12):2062-76. doi: 10.1002/stem.766. Stem Cells. 2011. PMID: 22038821 Free PMC article.
-
SNP-based genetic characterization of the Tulane National Primate Research Center's conventional and specific pathogen-free rhesus macaque (Macaca mulatta) populations.J Med Primatol. 2018 Feb;47(1):29-34. doi: 10.1111/jmp.12284. Epub 2017 Jun 21. J Med Primatol. 2018. PMID: 28639374 Free PMC article.
-
Kisspeptin-10 modulates the proliferation and differentiation of the rhesus monkey derived stem cell line: R366.4.ScientificWorldJournal. 2013 Nov 28;2013:135470. doi: 10.1155/2013/135470. eCollection 2013. ScientificWorldJournal. 2013. PMID: 24381507 Free PMC article.
-
iPSCs from people with MS can differentiate into oligodendrocytes in a homeostatic but not an inflammatory milieu.PLoS One. 2020 Jun 8;15(6):e0233980. doi: 10.1371/journal.pone.0233980. eCollection 2020. PLoS One. 2020. PMID: 32511247 Free PMC article.
-
Recent therapeutic strategies for spinal cord injury treatment: possible role of stem cells.Neurosurg Rev. 2012 Jul;35(3):293-311; discussion 311. doi: 10.1007/s10143-012-0385-2. Epub 2012 Apr 27. Neurosurg Rev. 2012. PMID: 22539011 Review.
References
-
- Baba H, Nakahira K, Morita N, Tanaka F, Akita H, Ikenaka K. GFAP gene expression during development of astrocyte. Dev. Neurosci. 1997;19:49–57. - PubMed
-
- Bani-Yaghoub M, Tremblay RG, Lei JX, Zhang D, Zurakowski B, Sandhu JK, Smith B, Ribecco-Lutkiewicz M, Kennedy J, Walker PR, Sikorska M. Role of Sox2 in the development of the mouse neocortex. Dev. Biol. 2006;295:52–66. - PubMed
-
- Barembaum M, Bronner-Fraser M. Early steps in neural crest specification. Semin. Cell Dev. Biol. 2005;16:642–646. - PubMed
-
- Baskin GB, Ratterree M, Davison BB, Falkenstein KP, Clarke MR, England JD, Vanier MT, Luzi P, Rafi MA, Wenger DA. Genetic galactocerebrosidase deficiency (globoid cell leukodystrophy, Krabbe disease) in rhesus monkeys (Macaca mulatta) Lab. Anim. Sci. 1998;48:476–482. - PubMed
-
- Benzing C, Segschneider M, Leinhaas A, Itskovitz-Eldor J, Brustle O. Neural conversion of human embryonic stem cell colonies in the presence of fibroblast growth factor-2. Neuroreport. 2006;17:1675–1681. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

