IGF-IR in neuroprotection and brain tumors
- PMID: 19273072
- PMCID: PMC2679154
- DOI: 10.2741/3249
IGF-IR in neuroprotection and brain tumors
Abstract
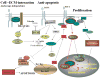
The IGF-IR is a multifunctional tyrosine kinase receptor involved in several biological processes including cell proliferation, differentiation, DNA repair, and cell survival. In the brain IGF-I plays a critical role during embryonic and early postnatal development. In the mature brain, IGF-I binding sites have been found in different regions of the brain, and multiple reports confirmed a strong neuroprotective action of the IGF-IR against different pro-apoptotic insults. When the IGF-IR signaling system is insufficiently deployed, either by low level of expression in elderly individuals, or by the inhibition associated with inflammatory cytokines, neuronal function and survival could be compromised. The examples of such CNS pathologies include HIV associated dementia, diabetic neuropathies, and Alzheimer's disease. On the other hand, elevated expression activity of the IGF-IR may support uncontrolled cell proliferation and protection from apoptosis. Probably the best example of the IGF-IR involvement in brain tumors is medulloblastomas in which functional cooperation between viral oncoprotein, JC virus large T-antigen, and IGF-IR has been recently established. Therefore, better understanding of the beneficial and potentially harmful aspects of the IGF-IR can be critical for the development of new clinical regimens against neurodegenerative disorders and brain tumors.
Figures




References
-
- Reiss K, Valentinis B, Tu X, Xu SQ, Baserga R. Molecular markers of IGF-I-mediated mitogenesis. Exp Cell Res. 1998;242:361–72. - PubMed
-
- Baserga R, Sell C, Porcu P, Rubini M. The role of the IGF-I receptor in the growth and transformation of mammalian cells. Cell Prolif. 1994;27:63–71. - PubMed
-
- Baserga R. The insulin-like growth factor I receptor: a key to tumor growth? Cancer Res. 1995;55:249–52. - PubMed
-
- LeRoith D, Baserga R, Helman L, Roberts CT., Jr Insulin-like growth factors and cancer. Ann Intern Med. 1995;122:54–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

