The role of saliva in tick feeding
- PMID: 19273185
- PMCID: PMC2785505
- DOI: 10.2741/3363
The role of saliva in tick feeding
Abstract
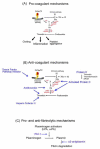
When attempting to feed on their hosts, ticks face the problem of host hemostasis (the vertebrate mechanisms that prevent blood loss), inflammation (that can produce itching or pain and thus initiate defensive behavior on their hosts) and adaptive immunity (by way of both cellular and humoral responses). Against these barriers, ticks evolved a complex and sophisticated pharmacological armamentarium, consisting of bioactive lipids and proteins, to assist blood feeding. Recent progress in transcriptome research has uncovered that hard ticks have hundreds of different proteins expressed in their salivary glands, the majority of which have no known function, and include many novel protein families (e.g., their primary structure is unique to ticks). This review will address the vertebrate mechanisms of these barriers as a guide to identify the possible targets of these large numbers of known salivary proteins with unknown function. We additionally provide a supplemental Table that catalogues over 3,500 putative salivary proteins from various tick species, which might assist the scientific community in the process of functional identification of these unique proteins. This supplemental file is accessble fromhttp://exon.niaid.nih.gov/transcriptome/tick_review/Sup-Table-1.xls.gz.
Figures

References
-
- Hoogstraal H, Aechlimann A. Tick host specificity. Bull Societe Entomol Suisse. 1982;55:5–32.
-
- Sauer JR. Acarine salivary glands -Physiological relationships. J Med Ent. 1977;14:1–9. - PubMed
-
- Bowman AS, Sauer JR. Tick salivary glands: function, physiology and future. Parasitology. 2004;129(Suppl):S67–81. - PubMed
-
- Gregson JD. Observations on the movement of fluids in the vicinity of the mouthparts of naturally feeding Dermacentor andersoni Stiles. Parasitol. 1967;57:1–8.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

