Endothelial signaling in paracellular and transcellular leukocyte transmigration
- PMID: 19273217
- PMCID: PMC2654604
- DOI: 10.2741/3395
Endothelial signaling in paracellular and transcellular leukocyte transmigration
Abstract
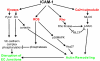
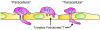
As the primary physical barrier between blood and tissue compartments within the body, blood vessel endothelial cells and integrity of the cell junctions connecting them must be carefully regulated to support leukocyte transendothelial migration only when necessary. Leukocytes utilize two independent routes across the endothelium: the paracellular route involves migration in-between adjacent endothelial cells and requires the transient disassembly of endothelial cell junctions, while the transcellular route occurs directly through an individual endothelial cell, likely requiring the formation of a channel or pore. In this review, I will first summarize the signaling events that are transduced by leukocyte engagement of endothelial cell-surface receptors like ICAM-1 and VCAM-1. Some of these signals include activation of GTPases, production of reactive oxygen species, and phosphorylation of target proteins. These signaling pathways converge to cause junctional disruption, cytoskeletal remodeling, and/or the membrane fusion events that are associated with leukocyte transendothelial migration. The review will conclude with a detailed discussion of the newly characterized transmigratory cup structure, and the recent advances made towards understanding the mechanisms of transcellular transendothelial migration.
Figures





References
-
- Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–6. - PubMed
-
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–14. - PubMed
-
- Wittchen ES, van Buul JD, Burridge K, Worthylake RA. Trading spaces: Rap, Rac, and Rho as architects of transendothelial migration. Curr Opin Hematol. 2005;12:14–21. - PubMed
-
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–89. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

