OST alpha-OST beta: a key membrane transporter of bile acids and conjugated steroids
- PMID: 19273238
- PMCID: PMC2694352
- DOI: 10.2741/3416
OST alpha-OST beta: a key membrane transporter of bile acids and conjugated steroids
Abstract
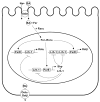
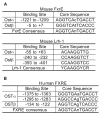
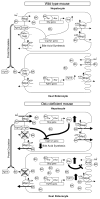
The organic solute and steroid transporter, Ost alpha-Ost beta, is an unusual heteromeric carrier that appears to play a central role in the transport of bile acids, conjugated steroids, and structurally-related molecules across the basolateral membrane of many epithelial cells. The transporter's substrate specificity, transport mechanism, tissue distribution, subcellular localization, transcriptional regulation, as well as the phenotype of the recently characterized Ost alpha-deficient mice all strongly support this model. In particular, the Ost alpha-deficient mice display a marked defect in intestinal bile acid and conjugated steroid absorption; a decrease in bile acid pool size and serum bile acid levels; altered intestinal, hepatic and renal disposition of known substrates of the transporter; and altered serum triglyceride, cholesterol, and glucose levels. Collectively, the data indicate that Ost alpha-Ost beta is essential for bile acid and sterol disposition, and suggest that the carrier may be involved in human conditions related to imbalances in bile acid or lipid homeostasis.
Figures




References
-
- Hofmann AF. Bile acids, cholesterol, gallstone calcification, and the enterohepatic circulation of bilirubin. Gastroenterology. 1999;116:1276–1277. - PubMed
-
- Trauner M, Boyer JL. Bile salt transporters: molecular characterization, function, and regulation. Physiol Rev. 2003;83:633–671. - PubMed
-
- Hofmann AF. Biliary secretion and excretion in health and disease: current concepts. Ann Hepatol. 2007;6:15–27. - PubMed
-
- Pellicoro A, Faber KN. Review article: the function and regulation of proteins involved in bile salt biosynthesis and transport. Aliment Pharmacol Ther. 2007;26:149–160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

