Transcription factor CTF1 acts as a chromatin domain boundary that shields human telomeric genes from silencing
- PMID: 19273604
- PMCID: PMC2668369
- DOI: 10.1128/MCB.00779-08
Transcription factor CTF1 acts as a chromatin domain boundary that shields human telomeric genes from silencing
Abstract
Telomeres are associated with chromatin-mediated silencing of genes in their vicinity. However, how epigenetic markers mediate mammalian telomeric silencing and whether specific proteins may counteract this effect are not known. We evaluated the ability of CTF1, a DNA- and histone-binding transcription factor, to prevent transgene silencing at human telomeres. CTF1 was found to protect a gene from silencing when its DNA-binding sites were interposed between the gene and the telomeric extremity, while it did not affect a gene adjacent to the telomere. Protein fusions containing the CTF1 histone-binding domain displayed similar activities, while mutants impaired in their ability to interact with the histone did not. Chromatin immunoprecipitation indicated the propagation of a hypoacetylated histone structure to various extents depending on the telomere. The CTF1 fusion protein was found to recruit the H2A.Z histone variant at the telomeric locus and to restore high histone acetylation levels to the insulated telomeric transgene. Histone lysine trimethylations were also increased on the insulated transgene, indicating that these modifications may mediate expression rather than silencing at human telomeres. Overall, these results indicate that transcription factors can act to delimit chromatin domain boundaries at mammalian telomeres, thereby blocking the propagation of a silent chromatin structure.
Figures


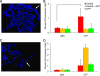
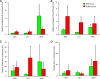
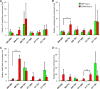
References
-
- Alevizopoulos, A., Y. Dusserre, M. Tsai-Pflugfelder, T. von der Weid, W. Wahli, and N. Mermod. 1995. A proline-rich TGF-beta-responsive transcriptional activator interacts with histone H3. Genes Dev. 93051-3066. - PubMed
-
- Barski, A., S. Cuddapah, K. Cui, T. Y. Roh, D. E. Schones, Z. Wang, G. Wei, I. Chepelev, and K. Zhao. 2007. High-resolution profiling of histone methylations in the human genome. Cell 129823-837. - PubMed
-
- Baur, J. A., Y. Zou, J. W. Shay, and W. E. Wright. 2001. Telomere position effect in human cells. Science 2922075-2077. - PubMed
-
- Bell, A. C., A. G. West, and G. Felsenfeld. 2001. Insulators and boundaries: versatile regulatory elements in the eukaryotic genome. Science 291447-450. - PubMed
-
- Benetti, R., M. Garcia-Cao, and M. A. Blasco. 2007. Telomere length regulates the epigenetic status of mammalian telomeres and subtelomeres. Nat. Genet. 39243-250. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
