Diverse cis factors controlling Alu retrotransposition: what causes Alu elements to die?
- PMID: 19273617
- PMCID: PMC2665774
- DOI: 10.1101/gr.089789.108
Diverse cis factors controlling Alu retrotransposition: what causes Alu elements to die?
Abstract
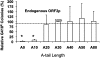
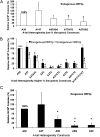
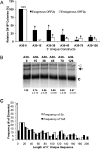
The human genome contains nearly 1.1 million Alu elements comprising roughly 11% of its total DNA content. Alu elements use a copy and paste retrotransposition mechanism that can result in de novo disease insertion alleles. There are nearly 900,000 old Alu elements from subfamilies S and J that appear to be almost completely inactive, and about 200,000 from subfamily Y or younger, which include a few thousand copies of the Ya5 subfamily which makes up the majority of current activity. Given the much higher copy number of the older Alu subfamilies, it is not known why all of the active Alu elements belong to the younger subfamilies. We present a systematic analysis evaluating the observed sequence variation in the different sections of an Alu element on retrotransposition. The length of the longest number of uninterrupted adenines in the A-tail, the degree of A-tail heterogeneity, the length of the 3' unique end after the A-tail and before the RNA polymerase III terminator, and random mutations found in the right monomer all modulate the retrotransposition efficiency. These changes occur over different evolutionary time frames. The combined impact of sequence changes in all of these regions explains why young Alus are currently causing disease through retrotransposition, and the old Alus have lost their ability to retrotranspose. We present a predictive model to evaluate the retrotransposition capability of individual Alu elements and successfully applied it to identify the first putative source element for a disease-causing Alu insertion in a patient with cystic fibrosis.
Figures

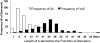



References
-
- Arcot S.S., Wang Z., Weber J.L., Deininger P.L., Batzer M.A. Alu repeats: A source for the genesis of primate microsatellites. Genomics. 1995;29:136–144. - PubMed
-
- Batzer M.A., Deininger P.L. Alu repeats and human genomic diversity. Nat. Rev. Genet. 2002;3:370–379. - PubMed
-
- Batzer M.A., Schmid C.W., Deininger P.L. Evolutionary analyses of repetitive DNA sequences. Methods Enzymol. 1993;224:213–232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
