Distinct DNA methylation patterns characterize differentiated human embryonic stem cells and developing human fetal liver
- PMID: 19273619
- PMCID: PMC2694474
- DOI: 10.1101/gr.088773.108
Distinct DNA methylation patterns characterize differentiated human embryonic stem cells and developing human fetal liver
Abstract
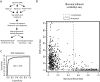
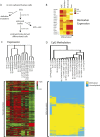
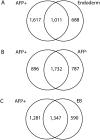
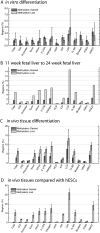
To investigate the role of DNA methylation during human development, we developed Methyl-seq, a method that assays DNA methylation at more than 90,000 regions throughout the genome. Performing Methyl-seq on human embryonic stem cells (hESCs), their derivatives, and human tissues allowed us to identify several trends during hESC and in vivo liver differentiation. First, differentiation results in DNA methylation changes at a minimal number of assayed regions, both in vitro and in vivo (2%-11%). Second, in vitro hESC differentiation is characterized by both de novo methylation and demethylation, whereas in vivo fetal liver development is characterized predominantly by demethylation. Third, hESC differentiation is uniquely characterized by methylation changes specifically at H3K27me3-occupied regions, bivalent domains, and low density CpG promoters (LCPs), suggesting that these regions are more likely to be involved in transcriptional regulation during hESC differentiation. Although both H3K27me3-occupied domains and LCPs are also regions of high variability in DNA methylation state during human liver development, these regions become highly unmethylated, which is a distinct trend from that observed in hESCs. Taken together, our results indicate that hESC differentiation has a unique DNA methylation signature that may not be indicative of in vivo differentiation.
Figures


References
-
- Antequera F., Boyes J., Bird A. High levels of de novo methylation and altered chromatin structure at CpG islands in cell lines. Cell. 1990;62:503–514. - PubMed
-
- Bernstein B.E., Mikkelsen T.S., Xie X., Kamal M., Huebert D.J., Cuff J., Fry B., Meissner A., Wernig M., Plath K., et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. - PubMed
-
- Bird A. CpG islands as gene markers in the vertebrate nucleus. Trends Genet. 1987;3:342–347.
-
- Bird A. DNA methylation patterns and epigenetic memory. Genes & Dev. 2002;16:6–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
