Inactivation of the glycoside hydrolase NagZ attenuates antipseudomonal beta-lactam resistance in Pseudomonas aeruginosa
- PMID: 19273679
- PMCID: PMC2687237
- DOI: 10.1128/AAC.01617-08
Inactivation of the glycoside hydrolase NagZ attenuates antipseudomonal beta-lactam resistance in Pseudomonas aeruginosa
Abstract
The overproduction of chromosomal AmpC beta-lactamase poses a serious challenge to the successful treatment of Pseudomonas aeruginosa infections with beta-lactam antibiotics. The induction of ampC expression by beta-lactams is mediated by the disruption of peptidoglycan (PG) recycling and the accumulation of cytosolic 1,6-anhydro-N-acetylmuramyl peptides, catabolites of PG recycling that are generated by an N-acetyl-beta-D-glucosaminidase encoded by nagZ (PA3005). In the absence of beta-lactams, ampC expression is repressed by three AmpD amidases encoded by ampD, ampDh2, and ampDh3, which act to degrade these 1,6-anhydro-N-acetylmuramyl peptide inducer molecules. The inactivation of ampD genes results in the stepwise upregulation of ampC expression and clinical resistance to antipseudomonal beta-lactams due to the accumulation of the ampC inducer anhydromuropeptides. To examine the role of NagZ on AmpC-mediated beta-lactam resistance in P. aeruginosa, we inactivated nagZ in P. aeruginosa PAO1 and in an isogenic triple ampD null mutant. We show that the inactivation of nagZ represses both the intrinsic beta-lactam resistance (up to 4-fold) and the high antipseudomonal beta-lactam resistance (up to 16-fold) that is associated with the loss of AmpD activity. We also demonstrate that AmpC-mediated resistance to antipseudomonal beta-lactams can be attenuated in PAO1 and in a series of ampD null mutants using a selective small-molecule inhibitor of NagZ. Our results suggest that the blockage of NagZ activity could provide a strategy to enhance the efficacies of beta-lactams against P. aeruginosa and other gram-negative organisms that encode inducible chromosomal ampC and to counteract the hyperinduction of ampC that occurs from the selection of ampD null mutations during beta-lactam therapy.
Figures


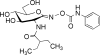
References
-
- Bartowsky, E., and S. Normark. 1991. Purification and mutant analysis of Citrobacter freundii AmpR, the regulator for chromosomal AmpC beta-lactamase. Mol. Microbiol. 5:1715-1725. - PubMed
-
- Clinical and Laboratory Standards Institute. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, sixth edition (M7-A6). Clinical and Laboratory Standards Institute, Wayne, PA.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Molecular Biology Databases

