PGC-1alpha expression decreases in the Alzheimer disease brain as a function of dementia
- PMID: 19273754
- PMCID: PMC3052997
- DOI: 10.1001/archneurol.2008.588
PGC-1alpha expression decreases in the Alzheimer disease brain as a function of dementia
Abstract
Objectives: To explore mechanisms through which altered peroxisome proliferator-activated receptor gamma coactivator 1alpha (PGC-1alpha) expression may influence Alzheimer disease (AD) amyloid neuropathology and to test the hypothesis that promotion of PGC-1alpha expression in neurons might be developed as a novel therapeutic strategy in AD.
Design: Case-control. Patients Human postmortem brain (hippocampal formation) samples from AD cases and age-matched non-AD cases.
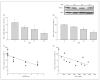
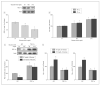
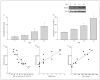
Results: Using genome-wide complementary DNA microarray analysis, we found that PGC-1alpha messenger RNA expression was significantly decreased as a function of progression of clinical dementia in the AD brain. Following confirmatory real-time polymerase chain reaction assay, we continued to explore the role of PGC-1alpha in clinical dementia and found that PGC-1alpha protein content was negatively associated with both AD-type neuritic plaque pathology and beta-amyloid (Abeta)(X-42) contents. Moreover, we found that the predicted elevation of amyloidogenic Abeta(1-42) and Abeta(1-40) peptide accumulation in embryonic cortico-hippocampal neurons derived from Tg2576 AD mice under hyperglycemic conditions (glucose level, 182-273 mg/dL) coincided with a dose-dependent attenuation in PGC-1alpha expression. Most importantly, we found that the reconstitution of exogenous PGC-1alpha expression in Tg2576 neurons attenuated the hyperglycemic-mediated beta-amyloidogenesis through mechanisms involving the promotion of the "nonamyloidogenic" alpha-secretase processing of amyloid precursor protein through the attenuation of the forkheadlike transcription factor 1 (FoxO3a) expression.
Conclusion: Therapeutic preservation of neuronal PGC-1alpha expression promotes the nonamyloidogenic processing of amyloid precursor protein precluding the generation of amyloidogenic Abeta peptides.
Figures

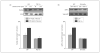

References
-
- Frölich L, Blum-Degen D, Bernstein HG, et al. Brain insulin and insulin receptors in aging and sporadic Alzheimer's disease. J Neural Transm. 1998;105(4-5):423–438. - PubMed
-
- Jagust WJ, Seab JP, Huesman RH, et al. Diminished glucose transport in Alzheimer's disease: dynamic PET studies. J Cereb Blood Flow Metab. 1991;11(2):323–330. - PubMed
-
- Minoshima S, Frey KA, Foster NL, Kuhl DE. Preserved pontine glucose metabolism in Alzheimer disease: a reference region for functional brain image (PET) analysis. J Comput Assist Tomogr. 1995;19(4):541–547. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

