Relative CO2/NH3 selectivities of AQP1, AQP4, AQP5, AmtB, and RhAG
- PMID: 19273840
- PMCID: PMC2664022
- DOI: 10.1073/pnas.0813231106
Relative CO2/NH3 selectivities of AQP1, AQP4, AQP5, AmtB, and RhAG
Abstract
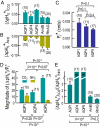
The water channel aquaporin 1 (AQP1) and certain Rh-family members are permeable to CO(2) and NH(3). Here, we use changes in surface pH (pH(S)) to assess relative CO(2) vs. NH(3) permeability of Xenopus oocytes expressing members of the AQP or Rh family. Exposed to CO(2) or NH(3), AQP1 oocytes exhibit a greater maximal magnitude of pH(S) change (DeltapH(S)) compared with day-matched controls injected with H(2)O or with RNA encoding SGLT1, NKCC2, or PepT1. With CO(2), AQP1 oocytes also have faster time constants for pH(S) relaxation (tau(pHs)). Thus, AQP1, but not the other proteins, conduct CO(2) and NH(3). Oocytes expressing rat AQP4, rat AQP5, human RhAG, or the bacterial Rh homolog AmtB also exhibit greater DeltapH(S)(CO(2)) and faster tau(pHs) compared with controls. Oocytes expressing AmtB and RhAG, but not AQP4 or AQP5, exhibit greater DeltapH(S)(NH(3)) values. Only AQPs exhibited significant osmotic water permeability (P(f)). We computed channel-dependent (*) DeltapH(S) or P(f) by subtracting values for H(2)O oocytes from those of channel-expressing oocytes. For the ratio DeltapH(S)(CO(2))*/P(f)*, the sequence was AQP5 > AQP1 congruent with AQP4. For DeltapH(S)(CO(2))*/DeltapH(S)(NH(3))*, the sequence was AQP4 congruent with AQP5 > AQP1 > AmtB > RhAG. Thus, each channel exhibits a characteristic ratio for indices of CO(2) vs. NH(3) permeability, demonstrating that, like ion channels, gas channels can exhibit selectivity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Waisbren SJ, et al. Unusual permeability properties of gastric gland cells. Nature. 1994;368:332–335. - PubMed
-
- Nakhoul NL, et al. Effect of expressing the water channel aquaporin-1 on the CO2 permeability of Xenopus oocytes. Am J Physiol. 1998;274:C543–C548. - PubMed
-
- Cooper GJ, Boron WF. Effect of pCMBS on CO2 permeability of Xenopus oocytes expressing aquaporin 1 or its C189S mutant. Am J Physiol. 1998;275:C1481–C1486. - PubMed
-
- Prasad GV, et al. Reconstituted aquaporin 1 water channels transport CO2 across membranes. J Biol Chem. 1998;273:33123–33126. - PubMed
-
- Uehlein N, et al. The tobacco aquaporin NtAQP1 is a membrane CO2 pore with physiological functions. Nature. 2003;425:734–737. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

