Core-shell hydrogel particles harvest, concentrate and preserve labile low abundance biomarkers
- PMID: 19274087
- PMCID: PMC2651577
- DOI: 10.1371/journal.pone.0004763
Core-shell hydrogel particles harvest, concentrate and preserve labile low abundance biomarkers
Abstract
Background: The blood proteome is thought to represent a rich source of biomarkers for early stage disease detection. Nevertheless, three major challenges have hindered biomarker discovery: a) candidate biomarkers exist at extremely low concentrations in blood; b) high abundance resident proteins such as albumin mask the rare biomarkers; c) biomarkers are rapidly degraded by endogenous and exogenous proteinases.
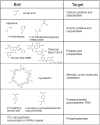
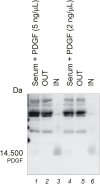
Methodology and principal findings: Hydrogel nanoparticles created with a N-isopropylacrylamide based core (365 nm)-shell (167 nm) and functionalized with a charged based bait (acrylic acid) were studied as a technology for addressing all these biomarker discovery problems, in one step, in solution. These harvesting core-shell nanoparticles are designed to simultaneously conduct size exclusion and affinity chromatography in solution. Platelet derived growth factor (PDGF), a clinically relevant, highly labile, and very low abundance biomarker, was chosen as a model. PDGF, spiked in human serum, was completely sequestered from its carrier protein albumin, concentrated, and fully preserved, within minutes by the particles. Particle sequestered PDGF was fully protected from exogenously added tryptic degradation. When the nanoparticles were added to a 1 mL dilute solution of PDGF at non detectable levels (less than 20 picograms per mL) the concentration of the PDGF released from the polymeric matrix of the particles increased within the detection range of ELISA and mass spectrometry. Beyond PDGF, the sequestration and protection from degradation for a series of additional very low abundance and very labile cytokines were verified.
Conclusions and significance: We envision the application of harvesting core-shell nanoparticles to whole blood for concentration and immediate preservation of low abundance and labile analytes at the time of venipuncture.
Conflict of interest statement
Figures








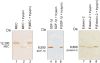
Similar articles
-
Multifunctional core-shell nanoparticles: discovery of previously invisible biomarkers.J Am Chem Soc. 2011 Nov 30;133(47):19178-88. doi: 10.1021/ja207515j. Epub 2011 Nov 3. J Am Chem Soc. 2011. PMID: 21999289 Free PMC article.
-
Nanoparticle technology: addressing the fundamental roadblocks to protein biomarker discovery.Curr Mol Med. 2010 Mar;10(2):133-41. doi: 10.2174/156652410790963268. Curr Mol Med. 2010. PMID: 20196732 Free PMC article. Review.
-
Hydrogel nanoparticle harvesting of plasma or urine for detecting low abundance proteins.J Vis Exp. 2014 Aug 7;(90):e51789. doi: 10.3791/51789. J Vis Exp. 2014. PMID: 25145492 Free PMC article.
-
Application of Hydrogel Nanoparticles for the Capture, Concentration, and Preservation of Low-Abundance Biomarkers.Methods Mol Biol. 2017;1606:103-113. doi: 10.1007/978-1-4939-6990-6_7. Methods Mol Biol. 2017. PMID: 28501996
-
Nanoparticle technology: amplifying the effective sensitivity of biomarker detection to create a urine test for hGH.Drug Test Anal. 2009 Sep;1(9-10):447-54. doi: 10.1002/dta.96. Drug Test Anal. 2009. PMID: 20355230 Free PMC article. Review.
Cited by
-
The use of hydrogel microparticles to sequester and concentrate bacterial antigens in a urine test for Lyme disease.Biomaterials. 2011 Feb;32(4):1157-66. doi: 10.1016/j.biomaterials.2010.10.004. Epub 2010 Oct 28. Biomaterials. 2011. PMID: 21035184 Free PMC article.
-
Substrate-baited nanoparticles: a catch and release strategy for enzyme recognition and harvesting.Small. 2012 Jul 9;8(13):2083-90. doi: 10.1002/smll.201200013. Epub 2012 Apr 24. Small. 2012. PMID: 22532510 Free PMC article.
-
Solid-phase extraction strategies to surmount body fluid sample complexity in high-throughput mass spectrometry-based proteomics.J Anal Methods Chem. 2015;2015:250131. doi: 10.1155/2015/250131. Epub 2015 Jan 27. J Anal Methods Chem. 2015. PMID: 25692071 Free PMC article. Review.
-
The use of Nanotrap particles technology in capturing HIV-1 virions and viral proteins from infected cells.PLoS One. 2014 May 12;9(5):e96778. doi: 10.1371/journal.pone.0096778. eCollection 2014. PLoS One. 2014. PMID: 24820173 Free PMC article.
-
The use of Nanotrap particles in the enhanced detection of Rift Valley fever virus nucleoprotein.PLoS One. 2015 May 28;10(5):e0128215. doi: 10.1371/journal.pone.0128215. eCollection 2015. PLoS One. 2015. PMID: 26020252 Free PMC article.
References
-
- Liotta LA, Ferrari M, Petricoin E. Clinical proteomics: written in blood. Nature. 2003;425:905. - PubMed
-
- Anderson NL, Anderson NG. The human plasma proteome: history, character, and diagnostic prospects. Mol Cell Proteomics. 2002;1:845–867. - PubMed
-
- Petricoin EF, Ardekani AM, Hitt BA, Levine PJ, Fusaro VA, et al. Use of proteomic patterns in serum to identify ovarian cancer. Lancet. 2002;359:572–577. - PubMed
-
- Petricoin EF, Belluco C, Araujo RP, Liotta LA. The blood peptidome: a higher dimension of information content for cancer biomarker discovery. Nat Rev Cancer. 2006;6:961–967. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

