Identification of ischemic regions in a rat model of stroke
- PMID: 19274095
- PMCID: PMC2652027
- DOI: 10.1371/journal.pone.0004764
Identification of ischemic regions in a rat model of stroke
Abstract
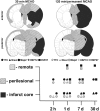
Background: Investigations following stroke first of all require information about the spatio-temporal dimension of the ischemic core as well as of perilesional and remote affected tissue. Here we systematically evaluated regions differently impaired by focal ischemia.
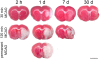
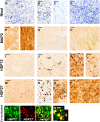
Methodology/principal findings: Wistar rats underwent a transient 30 or 120 min suture-occlusion of the middle cerebral artery (MCAO) followed by various reperfusion times (2 h, 1 d, 7 d, 30 d) or a permanent MCAO (1 d survival). Brains were characterized by TTC, thionine, and immunohistochemistry using MAP2, HSP72, and HSP27. TTC staining reliably identifies the infarct core at 1 d of reperfusion after 30 min MCAO and at all investigated times following 120 min and permanent MCAO. Nissl histology denotes the infarct core from 2 h up to 30 d after transient as well as permanent MCAO. Absent and attenuated MAP2 staining clearly identifies the infarct core and perilesional affected regions at all investigated times, respectively. HSP72 denotes perilesional areas in a limited post-ischemic time (1 d). HSP27 detects perilesional and remote impaired tissue from post-ischemic day 1 on. Furthermore a simultaneous expression of HSP72 and HSP27 in perilesional neurons was revealed.
Conclusions/significance: TTC and Nissl staining can be applied to designate the infarct core. MAP2, HSP72, and HSP27 are excellent markers not only to identify perilesional and remote areas but also to discriminate affected neuronal and glial populations. Moreover markers vary in their confinement to different reperfusion times. The extent and consistency of infarcts increase with prolonged occlusion of the MCA. Therefore interindividual infarct dimension should be precisely assessed by the combined use of different markers as described in this study.
Conflict of interest statement
Figures

References
-
- Witte OW, Bidmon HJ, Schiene K, Redecker C, Hagemann G. Functional differentiation of multiple perilesional zones after focal cerebral ischemia. J Cereb Blood Flow Metab. 2000;20:1149–1165. - PubMed
-
- Koizumi J, Yoshida Y, Nakazawa T, Ooneda G. Experimental studies of ischemic brain edema. 1. A new experimental model of cerebral embolism in rats in which recirculation can be introduced in the ischemic area. Jpn J Stroke. 1986;8:1–8.
-
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. - PubMed
-
- Nagasawa H, Kogure K. Correlation between cerebral blood flow and histologic changes in a new rat model of middle cerebral artery occlusion. Stroke. 1989;20:1037–1043. - PubMed
-
- Belayev L, Alonso OF, Busto R, Zhao W, Ginsberg MD. Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke. 1996;27:1616–1622. discussion 1623. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

