Nicotinic receptor-associated modulation of stimulatory and inhibitory neurotransmitters in NNK-induced adenocarcinoma of the lungs and pancreas
- PMID: 19274673
- PMCID: PMC3372983
- DOI: 10.1002/path.2542
Nicotinic receptor-associated modulation of stimulatory and inhibitory neurotransmitters in NNK-induced adenocarcinoma of the lungs and pancreas
Abstract
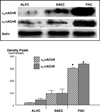
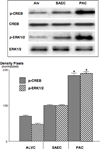
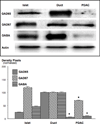
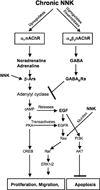
Small airway-derived pulmonary adenocarcinoma (PAC) and pancreatic ductal adenocarcinoma (PDAC) are among the most common human cancers and smoking is a risk factor for both. Emerging research has identified cAMP signalling stimulated by the stress neurotransmitters adrenaline and noradrenaline as an important stimulator of adenocarcinomas, including PAC and PDAC. The nicotine-derived nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) is a potent mutagen and the most powerful tobacco carcinogen. NNK is also an agonist for nicotinic acetylcholine receptors (nAChRs). Using hamster models of NNK-induced PAC and PDAC, we have tested the hypothesis that in analogy to chronic effects of nicotine in the brain, NNK may modulate the alpha(7)- and alpha(4)beta(2)nAChRs, causing an increase in stress neurotransmitters and a decrease in the inhibitory neurotransmitter gamma-aminobutyric acid (GABA). Immunoassays showed a significant increase in serum adrenaline/noradrenaline and increased intracellular cAMP in the cellular fraction of blood of NNK-treated hamsters. Western blots on microdissected control small airway epithelia, alveolar epithelia, pancreatic islet and pancreatic duct epithelia, and from NNK-induced PACs and PDACs showed that the GABA-synthesizing enzyme glutamate decarboxylase 65 (GAD65) and GABA were suppressed in NNK-induced PACs and PDACs. In contrast, protein expression of the alpha(7)nAChR, alpha(4)nAChR as well as p-CREB and p-ERK1/2 were up-regulated. These findings suggest that NNK-induced alterations in regulatory nAChRs may contribute to the development of smoking-associated PAC and PDAC by disturbing the balance between cancer-stimulating and -inhibiting neurotransmitters.
(c) 2009 Pathological Society of Great Britain and Ireland. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Conflict of interest: None to declare.
Figures




Similar articles
-
Prevention of pancreatic cancer by the beta-blocker propranolol.Anticancer Drugs. 2009 Jul;20(6):477-82. doi: 10.1097/CAD.0b013e32832bd1e3. Anticancer Drugs. 2009. PMID: 19387337 Free PMC article.
-
Nicotine stimulates pancreatic cancer xenografts by systemic increase in stress neurotransmitters and suppression of the inhibitory neurotransmitter gamma-aminobutyric acid.Carcinogenesis. 2009 Mar;30(3):506-11. doi: 10.1093/carcin/bgp010. Epub 2009 Jan 8. Carcinogenesis. 2009. PMID: 19131543 Free PMC article.
-
Gamma-aminobutyric acid, a potential tumor suppressor for small airway-derived lung adenocarcinoma.Carcinogenesis. 2008 Oct;29(10):1979-85. doi: 10.1093/carcin/bgn041. Epub 2008 Feb 28. Carcinogenesis. 2008. PMID: 18310090 Free PMC article.
-
Neurotransmitter receptors as central regulators of pancreatic cancer.Future Oncol. 2010 Feb;6(2):221-8. doi: 10.2217/fon.09.171. Future Oncol. 2010. PMID: 20146581 Free PMC article. Review.
-
Nitrosamines as nicotinic receptor ligands.Life Sci. 2007 May 30;80(24-25):2274-80. doi: 10.1016/j.lfs.2007.03.006. Epub 2007 Mar 19. Life Sci. 2007. PMID: 17459420 Free PMC article. Review.
Cited by
-
Pathologic cellular events in smoking-related pancreatitis.Cancers (Basel). 2015 Apr 29;7(2):723-35. doi: 10.3390/cancers7020723. Cancers (Basel). 2015. PMID: 25938854 Free PMC article. Review.
-
The pathobiological impact of cigarette smoke on pancreatic cancer development (review).Int J Oncol. 2012 Jul;41(1):5-14. doi: 10.3892/ijo.2012.1414. Epub 2012 Mar 23. Int J Oncol. 2012. PMID: 22446714 Free PMC article. Review.
-
Regulation of pancreatic cancer by neuropsychological stress responses: a novel target for intervention.Carcinogenesis. 2012 Jan;33(1):191-6. doi: 10.1093/carcin/bgr251. Epub 2011 Nov 9. Carcinogenesis. 2012. PMID: 22072614 Free PMC article.
-
Exploiting unique features of the gut-brain interface to combat gastrointestinal cancer.J Clin Invest. 2021 May 17;131(10):e143776. doi: 10.1172/JCI143776. J Clin Invest. 2021. PMID: 33998603 Free PMC article. Review.
-
Nicotine, IFN-γ and retinoic acid mediated induction of MUC4 in pancreatic cancer requires E2F1 and STAT-1 transcription factors and utilize different signaling cascades.Mol Cancer. 2012 Apr 26;11:24. doi: 10.1186/1476-4598-11-24. Mol Cancer. 2012. PMID: 22537161 Free PMC article.
References
-
- Devesa SS, Bray F, Vizcaino AP, Parkin DM. International lung cancer trends by histologic type: male:female differences diminishing and adenocarcinoma rates rising. Int J Cancer. 2005;117(2):294–299. - PubMed
-
- Talamini G, Bassi C, Falconi M, Sartori N, Salvia R, Rigo L, et al. Alcohol and smoking as risk factors in chronic pancreatitis and pancreatic cancer. Dig Dis Sci. 1999;44(7):1303–1311. - PubMed
-
- Hecht SS, Hoffmann D. N-nitroso compounds and tobacco-induced cancers in man. IARC Sci Publ. 1991;(105):54–61. - PubMed
-
- Hecht SS. Recent studies on mechanisms of bioactivation and detoxification of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), a tobacco-specific lung carcinogen. Crit Rev Toxicol. 1996;26(2):163–181. - PubMed
-
- Mitsudomi T, Viallet J, Mulshine JL, Linnoila RI, Minna JD, Gazdar AF. Mutations of ras genes distinguish a subset of non-small-cell lung cancer cell lines from small-cell lung cancer cell lines. Oncogene. 1991;6(8):1353–1362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

