Skeletally diverse small molecules using a build/couple/pair strategy
- PMID: 19275223
- PMCID: PMC2661762
- DOI: 10.1021/ol900173t
Skeletally diverse small molecules using a build/couple/pair strategy
Abstract
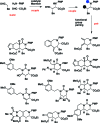
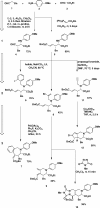
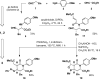
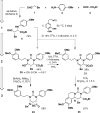
Intermolecular couplings of simple building blocks using catalytic, stereoselective cross-Mannich reactions followed by intramolecular functional group-pairing reactions of easily accessed variants of the Mannich products are explored as a route to skeletally diverse small molecules. The synthetic pathway yields products having 12 different skeletons using only three steps and has the potential to enable substantial stereochemical diversification in the future.
Figures
References
-
- Schreiber S. L. Nat. Chem. Biol. 2005, 1, 64–66. - PubMed
-
- Spiegel D. A.; Schroeder F. C.; Duvall J. R.; Schreiber S. L. J. Am. Chem. Soc. 2006, 128, 14766–14767. - PubMed
-
- Kumagai N.; Muncipinto G.; Schreiber S. L. Angew. Chem., Int. Ed. 2006, 45, 3635–3638. - PubMed
-
- List B.; Pojarliev P.; Biller W. T.; Martin H. J. J. Am. Chem. Soc. 2002, 124, 827–833. - PubMed
- Córdova A.; Watanabe S.; Tanaka F.; Notz W.; Barbas C. F. III. J. Am. Chem. Soc. 2002, 124, 1866–1867. - PubMed
- Hayashi Y.; Tsuboi W.; Ashimine I.; Urushima T.; Shoji M.; Sakai K. Angew. Chem., Int. Ed. 2003, 42, 3677–3680. - PubMed
- Notz W.; Tanaka F.; Watanabe S.; Chowdari N. S.; Turner J. M.; Thayumanavan R.; Barbas C. F. III. J. Org. Chem. 2003, 68, 9624–9634. - PubMed
- Chowdari N. S.; Suri J. T.; Barbas C. F. III. Org. Lett. 2004, 6, 2507–2510. - PubMed
- Ibrahem I.; Córdova A. Chem. Commun. 2006, 1760–1762. - PubMed
- Mitsumori S.; Zhang H.; Ha-Yeon Cheong P.; Houk K. N.; Tanaka F.; Barbas C. F. III. J. Am. Chem. Soc. 2006, 128, 1040–1041. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources