Variable impact on mortality of AIDS-defining events diagnosed during combination antiretroviral therapy: not all AIDS-defining conditions are created equal
- PMID: 19275498
- PMCID: PMC3032444
- DOI: 10.1086/597468
Variable impact on mortality of AIDS-defining events diagnosed during combination antiretroviral therapy: not all AIDS-defining conditions are created equal
Abstract
Background: The extent to which mortality differs following individual acquired immunodeficiency syndrome (AIDS)-defining events (ADEs) has not been assessed among patients initiating combination antiretroviral therapy.
Methods: We analyzed data from 31,620 patients with no prior ADEs who started combination antiretroviral therapy. Cox proportional hazards models were used to estimate mortality hazard ratios for each ADE that occurred in >50 patients, after stratification by cohort and adjustment for sex, HIV transmission group, number of antiretroviral drugs initiated, regimen, age, date of starting combination antiretroviral therapy, and CD4+ cell count and HIV RNA load at initiation of combination antiretroviral therapy. ADEs that occurred in <50 patients were grouped together to form a "rare ADEs" category.
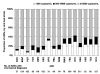
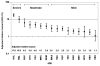
Results: During a median follow-up period of 43 months (interquartile range, 19-70 months), 2880 ADEs were diagnosed in 2262 patients; 1146 patients died. The most common ADEs were esophageal candidiasis (in 360 patients), Pneumocystis jiroveci pneumonia (320 patients), and Kaposi sarcoma (308 patients). The greatest mortality hazard ratio was associated with non-Hodgkin's lymphoma (hazard ratio, 17.59; 95% confidence interval, 13.84-22.35) and progressive multifocal leukoencephalopathy (hazard ratio, 10.0; 95% confidence interval, 6.70-14.92). Three groups of ADEs were identified on the basis of the ranked hazard ratios with bootstrapped confidence intervals: severe (non-Hodgkin's lymphoma and progressive multifocal leukoencephalopathy [hazard ratio, 7.26; 95% confidence interval, 5.55-9.48]), moderate (cryptococcosis, cerebral toxoplasmosis, AIDS dementia complex, disseminated Mycobacterium avium complex, and rare ADEs [hazard ratio, 2.35; 95% confidence interval, 1.76-3.13]), and mild (all other ADEs [hazard ratio, 1.47; 95% confidence interval, 1.08-2.00]).
Conclusions: In the combination antiretroviral therapy era, mortality rates subsequent to an ADE depend on the specific diagnosis. The proposed classification of ADEs may be useful in clinical end point trials, prognostic studies, and patient management.
Conflict of interest statement
Figures

References
-
- Gottlieb MS, Schroff R, Schanker HM, et al. Pneumocystis carinii pneumonia and mucosal candidiasis in previously healthy homosexual men: evidence of a new acquired cellular immunodeficiency. N Engl J Med. 1981;305:1425–31. - PubMed
-
- Hymes KB, Cheung T, Greene JB, et al. Kaposi’s sarcoma in homosexual men: a report of eight cases. Lancet. 1981;2:598–600. - PubMed
-
- Revision of the case definition of acquired immunodeficiency syndrome for national reporting: United States. Centers for Disease Control, Department of Health and Human Services. Ann Intern Med. 1985;103:402–3. - PubMed
-
- Leads from the MMWR: revision of the CDC surveillance case definition for acquired immunodeficiency syndrome. JAMA. 1987;258:1143–4. - PubMed
-
- 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41(RR-17):1–19. - PubMed

