Galectin-8 induces apoptosis in Jurkat T cells by phosphatidic acid-mediated ERK1/2 activation supported by protein kinase A down-regulation
- PMID: 19276072
- PMCID: PMC2675996
- DOI: 10.1074/jbc.M808949200
Galectin-8 induces apoptosis in Jurkat T cells by phosphatidic acid-mediated ERK1/2 activation supported by protein kinase A down-regulation
Abstract
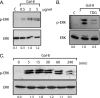
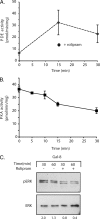
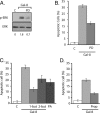
Galectins have been implicated in T cell homeostasis playing complementary pro-apoptotic roles. Here we show that galectin-8 (Gal-8) is a potent pro-apoptotic agent in Jurkat T cells inducing a complex phospholipase D/phosphatidic acid signaling pathway that has not been reported for any galectin before. Gal-8 increases phosphatidic signaling, which enhances the activity of both ERK1/2 and type 4 phosphodiesterases (PDE4), with a subsequent decrease in basal protein kinase A activity. Strikingly, rolipram inhibition of PDE4 decreases ERK1/2 activity. Thus Gal-8-induced PDE4 activation releases a negative influence of cAMP/protein kinase A on ERK1/2. The resulting strong ERK1/2 activation leads to expression of the death factor Fas ligand and caspase-mediated apoptosis. Several conditions that decrease ERK1/2 activity also decrease apoptosis, such as anti-Fas ligand blocking antibodies. In addition, experiments with freshly isolated human peripheral blood mononuclear cells, previously stimulated with anti-CD3 and anti-CD28, show that Gal-8 is pro-apoptotic on activated T cells, most likely on a subpopulation of them. Anti-Gal-8 autoantibodies from patients with systemic lupus erythematosus block the apoptotic effect of Gal-8. These results implicate Gal-8 as a novel T cell suppressive factor, which can be counterbalanced by function-blocking autoantibodies in autoimmunity.
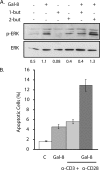
Figures


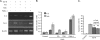
Similar articles
-
Galectin-8 binds specific beta1 integrins and induces polarized spreading highlighted by asymmetric lamellipodia in Jurkat T cells.Exp Cell Res. 2006 Feb 15;312(4):374-86. doi: 10.1016/j.yexcr.2005.10.025. Exp Cell Res. 2006. PMID: 16368432
-
Novel mechanism of cyclic AMP mediated extracellular signal regulated kinase activation in an intestinal cell line.Cell Signal. 2007 Jun;19(6):1221-8. doi: 10.1016/j.cellsig.2007.01.002. Epub 2007 Jan 21. Cell Signal. 2007. PMID: 17317103
-
FasL expression in cardiomyocytes activates the ERK1/2 pathway, leading to dilated cardiomyopathy and advanced heart failure.Clin Sci (Lond). 2016 Feb;130(4):289-99. doi: 10.1042/CS20150624. Epub 2015 Nov 13. Clin Sci (Lond). 2016. PMID: 26566650
-
Regulated expression of galectin-1 during T-cell activation involves Lck and Fyn kinases and signaling through MEK1/ERK, p38 MAP kinase and p70S6 kinase.Mol Cell Biochem. 2004 Dec;267(1-2):177-85. doi: 10.1023/b:mcbi.0000049376.50242.7f. Mol Cell Biochem. 2004. PMID: 15663199
-
Assay method for the antiproliferative activity of human galectin-9.2021 Jul 29 [updated 2022 Mar 16]. In: Nishihara S, Angata K, Aoki-Kinoshita KF, Hirabayashi J, editors. Glycoscience Protocols (GlycoPODv2) [Internet]. Saitama (JP): Japan Consortium for Glycobiology and Glycotechnology; 2021–. 2021 Jul 29 [updated 2022 Mar 16]. In: Nishihara S, Angata K, Aoki-Kinoshita KF, Hirabayashi J, editors. Glycoscience Protocols (GlycoPODv2) [Internet]. Saitama (JP): Japan Consortium for Glycobiology and Glycotechnology; 2021–. PMID: 37590616 Free Books & Documents. Review. No abstract available.
Cited by
-
Placental galectins regulate innate and adaptive immune responses in pregnancy.Front Immunol. 2022 Dec 28;13:1088024. doi: 10.3389/fimmu.2022.1088024. eCollection 2022. Front Immunol. 2022. PMID: 36643922 Free PMC article.
-
Expression and functions of galectin-7 in ovarian cancer.Oncotarget. 2014 Sep 15;5(17):7705-21. doi: 10.18632/oncotarget.2299. Oncotarget. 2014. PMID: 25277199 Free PMC article.
-
Alternative splicing in osteoclasts and Paget's disease of bone.BMC Med Genet. 2014 Aug 14;15:98. doi: 10.1186/s12881-014-0098-1. BMC Med Genet. 2014. PMID: 25115182 Free PMC article.
-
Lipid metabolism, apoptosis and cancer therapy.Int J Mol Sci. 2015 Jan 2;16(1):924-49. doi: 10.3390/ijms16010924. Int J Mol Sci. 2015. PMID: 25561239 Free PMC article. Review.
-
Galectins-Potential Therapeutic Targets for Neurodegenerative Disorders.Int J Mol Sci. 2022 Sep 20;23(19):11012. doi: 10.3390/ijms231911012. Int J Mol Sci. 2022. PMID: 36232314 Free PMC article. Review.
References
-
- Rabinovich, G. A., Liu, F. T., Hirashima, M., and Anderson, A. (2007) Scand. J. Immunol. 66 143–158 - PubMed
-
- Leffler, H., Carlsson, S., Hedlund, M., Qian, Y., and Poirier, F. (2004) Glycoconj. J. 19 433–440 - PubMed
-
- Cooper, D. N., and Barondes, S. H. (1999) Glycobiology 9 979–984 - PubMed
-
- Perillo, N. L., Marcus, M. E., and Baum, L. G. (1998) J. Mol. Med. 76 402–412 - PubMed
-
- Rabinovich, G. A. (1999) Cell Death Differ. 6 711–721 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

