Matrix metalloproteinase-2-deficient fibroblasts exhibit an alteration in the fibrotic response to connective tissue growth factor/CCN2 because of an increase in the levels of endogenous fibronectin
- PMID: 19276073
- PMCID: PMC2679456
- DOI: 10.1074/jbc.M807352200
Matrix metalloproteinase-2-deficient fibroblasts exhibit an alteration in the fibrotic response to connective tissue growth factor/CCN2 because of an increase in the levels of endogenous fibronectin
Abstract
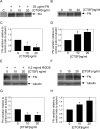
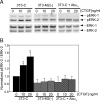
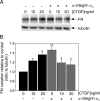
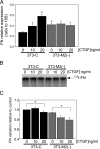
Matrix metalloproteinase-2 (MMP-2) is an important extracellular matrix remodeling enzyme, and it has been involved in different fibrotic disorders. The connective tissue growth factor (CTGF/CCN2), which is increased in these pathologies, induces the production of extracellular matrix proteins. To understand the fibrotic process observed in diverse pathologies, we analyzed the fibroblast response to CTGF when MMP-2 activity is inhibited. CTGF increased fibronectin (FN) amount, MMP-2 mRNA expression, and gelatinase activity in 3T3 cells. When MMP-2 activity was inhibited either by the metalloproteinase inhibitor GM-6001 or in MMP-2-deficient fibroblasts, an increase in the basal amount of FN together with a decrease of its levels in response to CTGF was observed. This paradoxical effect could be explained by the fact that the excess of FN could block the access to other ligands, such as CTGF, to integrins. This effect was emulated in fibroblasts by adding exogenous FN or RGDS peptides or using anti-integrin alpha(V) subunit-blocking antibodies. Additionally, in MMP-2-deficient cells CTGF did not induce the formation of stress fibers, focal adhesion sites, and ERK phosphorylation. Anti-integrin alpha(V) subunit-blocking antibodies inhibited ERK phosphorylation in control cells. Finally, in MMP-2-deficient cells, FN mRNA expression was not affected by CTGF, but degradation of (125)I-FN was increased. These results suggest that expression, regulation, and activity of MMP-2 can play an important role in the initial steps of fibrosis and shows that FN levels can regulate the cellular response to CTGF.
Figures


Similar articles
-
Connective tissue growth factor increases matrix metalloproteinase-2 and suppresses tissue inhibitor of matrix metalloproteinase-2 production by cultured renal interstitial fibroblasts.Wound Repair Regen. 2007 Nov-Dec;15(6):817-24. doi: 10.1111/j.1524-475X.2007.00284.x. Wound Repair Regen. 2007. PMID: 18028129
-
Expression of CTGF/CCN2 in response to LPA is stimulated by fibrotic extracellular matrix via the integrin/FAK axis.Am J Physiol Cell Physiol. 2018 Apr 1;314(4):C415-C427. doi: 10.1152/ajpcell.00013.2017. Epub 2017 Dec 27. Am J Physiol Cell Physiol. 2018. PMID: 29351412
-
CTGF is increased in basal deposits and regulates matrix production through the ERK (p42/p44mapk) MAPK and the p38 MAPK signaling pathways.Invest Ophthalmol Vis Sci. 2009 Apr;50(4):1903-10. doi: 10.1167/iovs.08-2383. Epub 2008 Nov 14. Invest Ophthalmol Vis Sci. 2009. PMID: 19011018 Free PMC article.
-
Transcriptional profiling of the scleroderma fibroblast reveals a potential role for connective tissue growth factor (CTGF) in pathological fibrosis.Keio J Med. 2004 Jun;53(2):74-7. doi: 10.2302/kjm.53.74. Keio J Med. 2004. PMID: 15247510 Review.
-
Regulation of hepatic stellate cells by connective tissue growth factor.Front Biosci (Landmark Ed). 2012 Jun 1;17(7):2495-507. doi: 10.2741/4067. Front Biosci (Landmark Ed). 2012. PMID: 22652794 Review.
Cited by
-
Loss of neuron-astroglial interaction rapidly induces protective CNTF expression after stroke in mice.J Neurosci. 2012 Jul 4;32(27):9277-87. doi: 10.1523/JNEUROSCI.1746-12.2012. J Neurosci. 2012. PMID: 22764235 Free PMC article.
-
Dual roles of tumour cells-derived matrix metalloproteinase 2 on brain tumour growth and invasion.Br J Cancer. 2017 Dec 5;117(12):1828-1836. doi: 10.1038/bjc.2017.362. Epub 2017 Oct 24. Br J Cancer. 2017. PMID: 29065106 Free PMC article.
-
Integrin expression and function in the response of primary culture hepatic stellate cells to connective tissue growth factor (CCN2).J Cell Mol Med. 2011 May;15(5):1087-95. doi: 10.1111/j.1582-4934.2010.01072.x. Epub 2010 Apr 19. J Cell Mol Med. 2011. PMID: 20406330 Free PMC article.
-
Efficacy of epidermal growth factor in suppressing inflammation and proliferation in pterygial fibroblasts through interactions with microenvironmental M1 macrophages.Sci Rep. 2024 Sep 30;14(1):22601. doi: 10.1038/s41598-024-74413-5. Sci Rep. 2024. PMID: 39349715 Free PMC article.
-
Regulatory mechanisms of anthrax toxin receptor 1-dependent vascular and connective tissue homeostasis.Matrix Biol. 2015 Mar;42:56-73. doi: 10.1016/j.matbio.2014.12.002. Epub 2015 Jan 5. Matrix Biol. 2015. PMID: 25572963 Free PMC article.
References
-
- Werb, Z. (1997) Cell 91 439-442 - PubMed
-
- Birkedal-Hansen, H. (1995) Curr. Opin. Cell Biol. 7 728-735 - PubMed
-
- Balcerzak, D., Querengesser, L., Dixon, W. T., and Baracos, V. E. (2001) J. Anim. Sci. 79 94-107 - PubMed
-
- Murphy, G., and Docherty, A. J. (1992) Am. J. Respir. Cell Mol. Biol. 7 120-125 - PubMed
-
- Strongin, A. Y., Collier, I., Bannikov, G., Marmer, B. L., Grant, G. A., and Goldberg, G. I. (1995) J. Biol. Chem. 270 5331-5338 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

