PapA3 is an acyltransferase required for polyacyltrehalose biosynthesis in Mycobacterium tuberculosis
- PMID: 19276083
- PMCID: PMC2676004
- DOI: 10.1074/jbc.M809088200
PapA3 is an acyltransferase required for polyacyltrehalose biosynthesis in Mycobacterium tuberculosis
Abstract
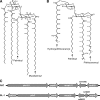
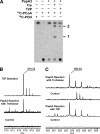
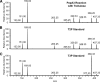
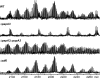
Mycobacterium tuberculosis possesses an unusual cell wall that is replete with virulence-enhancing lipids. One cell wall molecule unique to pathogenic M. tuberculosis is polyacyltrehalose (PAT), a pentaacylated, trehalose-based glycolipid. Little is known about the biosynthesis of PAT, although its biosynthetic gene cluster has been identified and found to resemble that of the better studied M. tuberculosis cell wall component sulfolipid-1. In this study, we sought to elucidate the function of papA3, a gene from the PAT locus encoding a putative acyltransferase. To determine whether PapA3 participates in PAT assembly, we expressed the protein heterologously and evaluated its acyltransferase activity in vitro. The purified enzyme catalyzed the sequential esterification of trehalose with two palmitoyl groups, generating a diacylated product similar to the 2,3-diacyltrehalose glycolipids of M. tuberculosis. Notably, PapA3 was selective for trehalose; no activity was observed with other structurally related disaccharides. Disruption of the papA3 gene from M. tuberculosis resulted in the loss of PAT from bacterial lipid extracts. Complementation of the mutant strain restored PAT production, demonstrating that PapA3 is essential for the biosynthesis of this glycolipid in vivo. Furthermore, we determined that the PAT biosynthetic machinery has no cross-talk with that for sulfolipid-1 despite their related structures.
Figures
Similar articles
-
The rv1184c locus encodes Chp2, an acyltransferase in Mycobacterium tuberculosis polyacyltrehalose lipid biosynthesis.J Bacteriol. 2015 Jan 1;197(1):201-10. doi: 10.1128/JB.02015-14. Epub 2014 Oct 20. J Bacteriol. 2015. PMID: 25331437 Free PMC article.
-
PapA1 and PapA2 are acyltransferases essential for the biosynthesis of the Mycobacterium tuberculosis virulence factor sulfolipid-1.Proc Natl Acad Sci U S A. 2007 Jul 3;104(27):11221-6. doi: 10.1073/pnas.0611649104. Epub 2007 Jun 25. Proc Natl Acad Sci U S A. 2007. PMID: 17592143 Free PMC article.
-
Biosynthesis and translocation of unsulfated acyltrehaloses in Mycobacterium tuberculosis.J Biol Chem. 2014 Oct 3;289(40):27952-65. doi: 10.1074/jbc.M114.581199. Epub 2014 Aug 14. J Biol Chem. 2014. PMID: 25124040 Free PMC article.
-
Genetics of Mycobacterial Trehalose Metabolism.Microbiol Spectr. 2014 Jun;2(3). doi: 10.1128/microbiolspec.MGM2-0002-2013. Microbiol Spectr. 2014. PMID: 26103976 Review.
-
Lipid transport in Mycobacterium tuberculosis and its implications in virulence and drug development.Biochem Pharmacol. 2015 Aug 1;96(3):159-67. doi: 10.1016/j.bcp.2015.05.001. Epub 2015 May 16. Biochem Pharmacol. 2015. PMID: 25986884 Review.
Cited by
-
Elucidation and chemical modulation of sulfolipid-1 biosynthesis in Mycobacterium tuberculosis.J Biol Chem. 2012 Mar 9;287(11):7990-8000. doi: 10.1074/jbc.M111.315473. Epub 2011 Dec 22. J Biol Chem. 2012. PMID: 22194604 Free PMC article.
-
Synthesis and in Vitro Characterization of Trehalose-Based Inhibitors of Mycobacterial Trehalose 6-Phosphate Phosphatases.Chembiochem. 2019 Jan 18;20(2):260-269. doi: 10.1002/cbic.201800551. Epub 2018 Dec 20. Chembiochem. 2019. PMID: 30402996 Free PMC article.
-
Genetics of Capsular Polysaccharides and Cell Envelope (Glyco)lipids.Microbiol Spectr. 2014;2(4):14. doi: 10.1128/microbiolspec.MGM2-0021-2013. Microbiol Spectr. 2014. PMID: 25485178 Free PMC article.
-
Comparison of the membrane proteome of virulent Mycobacterium tuberculosis and the attenuated Mycobacterium bovis BCG vaccine strain by label-free quantitative proteomics.J Proteome Res. 2013 Dec 6;12(12):5463-74. doi: 10.1021/pr400334k. Epub 2013 Oct 28. J Proteome Res. 2013. PMID: 24093440 Free PMC article.
-
Sulfolipid-1 biosynthesis restricts Mycobacterium tuberculosis growth in human macrophages.ACS Chem Biol. 2012 May 18;7(5):863-70. doi: 10.1021/cb200311s. Epub 2012 Feb 24. ACS Chem Biol. 2012. PMID: 22360425 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

