Calmodulin kinase II is required for fight or flight sinoatrial node physiology
- PMID: 19276108
- PMCID: PMC2667018
- DOI: 10.1073/pnas.0806422106
Calmodulin kinase II is required for fight or flight sinoatrial node physiology
Abstract
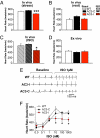
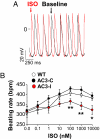
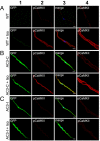
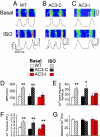
The best understood "fight or flight" mechanism for increasing heart rate (HR) involves activation of a cyclic nucleotide-gated ion channel (HCN4) by beta-adrenergic receptor (betaAR) agonist stimulation. HCN4 conducts an inward "pacemaker" current (I(f)) that increases the sinoatrial nodal (SAN) cell membrane diastolic depolarization rate (DDR), leading to faster SAN action potential generation. Surprisingly, HCN4 knockout mice were recently shown to retain physiological HR increases with isoproterenol (ISO), suggesting that other I(f)-independent pathways are critical to SAN fight or flight responses. The multifunctional Ca(2+) and calmodulin-dependent protein kinase II (CaMKII) is a downstream signal in the betaAR pathway that activates Ca(2+) homeostatic proteins in ventricular myocardium. Mice with genetic, myocardial and SAN cell CaMKII inhibition have significantly slower HRs than controls during stress, leading us to hypothesize that CaMKII actions on SAN Ca(2+) homeostasis are critical for betaAR agonist responses in SAN. Here we show that CaMKII mediates ISO HR increases by targeting SAN cell Ca(2+) homeostasis. CaMKII inhibition prevents ISO effects on SAN Ca(2+) uptake and release from intracellular sarcoplasmic reticulum (SR) stores that are necessary for increasing DDR. CaMKII inhibition has no effect on the ISO response in SAN cells when SR Ca(2+) release is disabled and CaMKII inhibition is only effective at slowing HRs during betaAR stimulation. These studies show the tightly coupled, but previously unanticipated, relationship of CaMKII to the betaAR pathway in fight or flight physiology and establish CaMKII as a critical signaling molecule for physiological HR responses to catecholamines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Lyashkov AE, et al. Calcium cycling protein density and functional importance to automaticity of isolated sinoatrial nodal cells are independent of cell size. Circ Res. 2007;100:1723–1731. - PubMed
-
- Rigg L, Heath BM, Cui Y, Terrar DA. Localisation and functional significance of ryanodine receptors during beta-adrenoceptor stimulation in the guinea-pig sino-atrial node. Cardiovasc Res. 2000;48:254–264. - PubMed
-
- Zhang R, et al. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med. 2005;11:409–417. - PubMed
-
- Curran J, Hinton MJ, Rios E, Bers DM, Shannon TR. {beta}-Adrenergic enhancement of sarcoplasmic reticulum calcium leak in cardiac myocytes is mediated by calcium/calmodulin-dependent protein kinase. Circ Res. 2007;100:391–398. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL062494/HL/NHLBI NIH HHS/United States
- R01 HL079031/HL/NHLBI NIH HHS/United States
- R01 HL 090905/HL/NHLBI NIH HHS/United States
- R01 HL096652/HL/NHLBI NIH HHS/United States
- R01 HL 70250/HL/NHLBI NIH HHS/United States
- R01 HL070250/HL/NHLBI NIH HHS/United States
- R01 HL089598/HL/NHLBI NIH HHS/United States
- R01 HL117641/HL/NHLBI NIH HHS/United States
- R01 HL 079031/HL/NHLBI NIH HHS/United States
- R01 HL083422/HL/NHLBI NIH HHS/United States
- R01 HL 089598/HL/NHLBI NIH HHS/United States
- R01 HL084583/HL/NHLBI NIH HHS/United States
- R01 HL090905/HL/NHLBI NIH HHS/United States
- R01 HL 62494/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

