Immune dysregulation in severe influenza
- PMID: 19276177
- PMCID: PMC2698588
- DOI: 10.1189/jlb.1108710
Immune dysregulation in severe influenza
Abstract
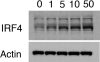
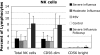
Among previously healthy children with severe influenza, the mechanisms leading to increased pathology are not understood. We hypothesized that children with severe influenza would have high levels of circulating cytokines. To examine this, we recruited patients with severe influenza and examined plasma cytokine levels as well as the ability of peripheral blood cells to respond to stimuli. Ten patients with severe influenza were enrolled during the 2005-2007 influenza seasons. We evaluated plasma cytokine levels, circulating NK cells, and responses to TLR ligands during the illness. We compared these patients with five patients with moderate influenza, six patients with respiratory syncytial virus (RSV), and 24 noninfected controls. Patients with influenza showed depressed responses to TLR ligands when compared with RSV patients and healthy controls (P<0.05). These normalized when retested during a convalescent phase. Plasma levels of IL-6, IL-12, and IFN- were elevated in influenza patients compared with controls (P<0.05). A compromised ability to produce TNF- was reproduced by in vitro infection, and the magnitude of the effect correlated with the multiplicity of infection and induction of IFN regulatory factor 4 expression. Aberrant, systemic, innate responses to TLR ligands during influenza infection may be a consequence of specific viral attributes such as a high inoculum or rapid replication and may underlie the known susceptibility of influenza-infected patients to secondary bacterial infections.
Figures
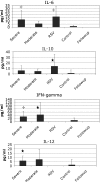
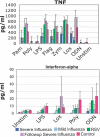
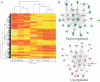
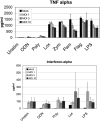
References
-
- Sabin A B. Mortality from pneumonia and risk conditions during influenza epidemics. High influenza morbidity during nonepidemic years. JAMA. 1977;237:2823–2828. - PubMed
-
- Glezen W P. Considerations of the risk of influenza in children and indications for prophylaxis. Rev Infect Dis. 1980;2:408–420. - PubMed
-
- Izurieta H S, Thompson W W, Kramarz P, Shay D K, Davis R L, DeStefano F, Black S, Shinefield H, Fukuda K. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med. 2000;342:232–239. - PubMed
-
- Ampofo K, Gesteland P H, Bender J, Mills M, Daly J, Samore M, Byington C, Pavia A T, Srivastava R. Epidemiology, complications, and cost of hospitalization in children with laboratory-confirmed influenza infection. Pediatrics. 2006;118:2409–2417. - PubMed
-
- Guarner J, Paddock C D, Shieh W J, Packard M M, Patel M, Montague J L, Uyeki T M, Bhat N, Balish A, Lindstrom S, Klimov A, Zaki S R. Histopathologic and immunohistochemical features of fatal influenza virus infection in children during the 2003–2004 season. Clin Infect Dis. 2006;43:132–140. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

